Abstract
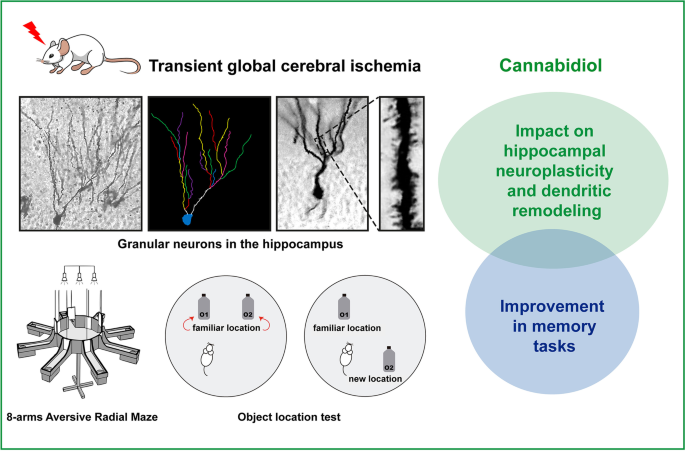
Evidence for the clinical use of neuroprotective drugs for the treatment of cerebral ischemia (CI) is still greatly limited. Spatial/temporal disorientation and cognitive dysfunction are among the most prominent long-term sequelae of CI. Cannabidiol (CBD) is a non-psychotomimetic constituent of Cannabis sativa that exerts neuroprotective effects against experimental CI. The present study investigated possible neuroprotective mechanisms of action of CBD on spatial memory impairments that are caused by transient global cerebral ischemia (TGCI) in rats. Hippocampal synaptic plasticity is a fundamental mechanism of learning and memory. Thus, we also evaluated the impact of CBD on neuroplastic changes in the hippocampus after TGCI. Wistar rats were trained to learn an eight-arm aversive radial maze (AvRM) task and underwent either sham or TGCI surgery. The animals received vehicle or 10 mg/kg CBD (i.p.) 30 min before surgery, 3 h after surgery, and then once daily for 14 days. On days 7 and 14, we performed a retention memory test. Another group of rats that received the same pharmacological treatment was tested in the object location test (OLT). Brains were removed and processed to assess neuronal degeneration, synaptic protein levels, and dendritic remodeling in the hippocampus. Cannabidiol treatment attenuated ischemia-induced memory deficits. In rats that were subjected to TGCI, CBD attenuated hippocampal CA1 neurodegeneration and increased brain-derived neurotrophic factor levels. Additionally, CBD protected neurons against the deleterious effects of TGCI on dendritic spine number and the length of dendritic arborization. These results suggest that the neuroprotective effects of CBD against TGCI-induced memory impairments involve changes in synaptic plasticity in the hippocampus.
Graphical abstract







Similar content being viewed by others
Data Availability
Experimental data will be made available under reasonable request.
References
Horstmann A, Frisch S, Jentzsch RT, Müller K, Villringer A, Schroeter ML (2010) Resuscitating the heart but losing the brain: brain atrophy in the aftermath of cardiac arrest. Neurology 74(4):306–312. https://doi.org/10.1212/WNL.0b013e3181cbcd6f
Sekhon MS, Ainslie PN, Griesdale DE (2017) Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care 21(1):90. https://doi.org/10.1186/s13054-017-1670-9
Canese R, Podo F, Fortuna S, Lorenzini P, Michalek H (1997) Transient global brain ischemia in the rat: spatial distribution, extension, and evolution of lesions evaluated by magnetic resonance imaging. MAGMA 5(2):139–149. https://doi.org/10.1007/BF02592245
Wahul AB, Joshi PC, Kumar A, Chakravarty S (2018) Transient global cerebral ischemia differentially affects cortex, striatum and hippocampus in Bilateral Common Carotid Arterial occlusion (BCCAo) mouse model. J Chem Neuroanat 92:1–15. https://doi.org/10.1016/j.jchemneu.2018.04.006
Petito CK, Feldmann E, Pulsinelli WA, Plum F (1987) Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology 37(8):1281–1286. https://doi.org/10.1212/wnl.37.8.1281
Ng T, Graham DI, Adams JH, Ford I (1989) Changes in the hippocampus and the cerebellum resulting from hypoxic insults: frequency and distribution. Acta Neuropathol 78(4):438–443. https://doi.org/10.1007/BF00688181
Pulsinelli WA, Brierley JB, Plum F (1982) Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 11(5):491–498. https://doi.org/10.1002/ana.410110509
Kirino T (2000) Delayed neuronal death. Neuropathology 20:S95–S97. https://doi.org/10.1046/j.1440-1789.2000.00306.x
Anderson CA, Arciniegas DB (2010) Cognitive sequelae of hypoxic-ischemic brain injury: a review. NeuroRehabilitation 26(1):47–63. https://doi.org/10.3233/NRE-2010-0535
Dirnagl U, Endres M (2014) Found in translation: preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke 45(5):1510–1518. https://doi.org/10.1161/STROKEAHA.113.004075
Lapchak PA, Zhang JH (2017) The high cost of stroke and stroke cytoprotection research. Transl Stroke Res 8(4):307–317. https://doi.org/10.1007/s12975-016-0518-y
Martin RC, Gaston TE, Thompson M, Ampah SB, Cutter G, Bebin EM, Szaflarski JP (2019) Cognitive functioning following long-term cannabidiol use in adults with treatment-resistant epilepsy. Epilepsy Behav 97:105–110. https://doi.org/10.1016/j.yebeh.2019.04.044
Gaston TE, Allendorfer JB, Nair S, Bebin EM, Grayson LP, Martin RC, Szaflarski JP, Program UABCBD (2020) Effects of highly purified cannabidiol (CBD) on fMRI of working memory in treatment-resistant epilepsy. Epilepsy Behav 112:107358. https://doi.org/10.1016/j.yebeh.2020.107358
Metternich B, Wagner K, Geiger MJ, Hirsch M, Schulze-Bonhage A, Klotz KA (2021) Cognitive and behavioral effects of cannabidiol in patients with treatment-resistant epilepsy. Epilepsy Behav 114(Pt A):107558. https://doi.org/10.1016/j.yebeh.2020.107558
Osborne AL, Solowij N, Weston-Green K (2017) A systematic review of the effect of cannabidiol on cognitive function: relevance to schizophrenia. Neurosci Biobehav Rev 72:310–324. https://doi.org/10.1016/j.neubiorev.2016.11.012
Pazos MR, Cinquina V, Gómez A, Layunta R, Santos M, Fernández-Ruiz J, Martínez Orgado J (2012) Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 63(5):776–783. https://doi.org/10.1016/j.neuropharm.2012.05.034
Schiavon AP, Soares LM, Bonato JM, Milani H, Guimarães FS, Weffort de Oliveira RM (2014) Protective effects of cannabidiol against hippocampal cell death and cognitive impairment induced by bilateral common carotid artery occlusion in mice. Neurotox Res 26(4):307–316. https://doi.org/10.1007/s12640-014-9457-0
Mori MA, Meyer E, Soares LM, Milani H, Guimarães FS, de Oliveira RMW (2017) Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog Neuropsychopharmacol Biol Psychiatry 75:94–105. https://doi.org/10.1016/j.pnpbp.2016.11.005
Fogaça MV, Campos AC, Coelho LD, Duman RS, Guimarães FS (2018) The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology 135:22–33. https://doi.org/10.1016/j.neuropharm.2018.03.001
Hayakawa K, Mishima K, Irie K, Hazekawa M, Mishima S, Fujioka M, Orito K, Egashira N, Katsurabayashi S, Takasaki K, Iwasaki K, Fujiwara M (2008) Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology 55(8):1280–1286. https://doi.org/10.1016/j.neuropharm.2008.06.040
Pulsinelli WA, Brierley JB (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 10(3):267–272. https://doi.org/10.1161/01.str.10.3.267
de Oliveira DV, Bernardi TC, de Melo SR, Godinho J, de Oliveira RMW, Milani H (2019) Postischemic fish oil treatment restores dendritic integrity and synaptic proteins levels after transient, global cerebral ischemia in rats. J Chem Neuroanat 101:101683. https://doi.org/10.1016/j.jchemneu.2019.101683
Seif el Nasr M, Nuglisch J, Krieglstein J (1992) Prevention of ischemia-induced cerebral hypothermia by controlling the environmental temperature. J Pharmacol Toxicol Methods 27(1):23–26. https://doi.org/10.1016/1056-8719(92)90016-t
Fernandes JS, Mori MA, Ekuni R, Oliveira RM, Milani H (2008) Long-term treatment with fish oil prevents memory impairments but not hippocampal damage in rats subjected to transient, global cerebral ischemia. Nutr Res 28(11):798–808. https://doi.org/10.1016/j.nutres.2008.09.004
Ennaceur A, Michalikova S, Bradford A, Ahmed S (2005) Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav Brain Res 159(2):247–266. https://doi.org/10.1016/j.bbr.2004.11.006
Akkerman S, Blokland A, Reneerkens O, van Goethem NP, Bollen E, Gijselaers HJ, Lieben CK, Steinbusch HW, Prickaerts J (2012) Object recognition testing: methodological considerations on exploration and discrimination measures. Behav Brain Res 232:335–347. https://doi.org/10.1016/j.bbr.2012.03.022
Gull S, Ingrisch I, Tausch S, Witte OW, Schmidt S (2015) Consistent and reproducible staining of glia by a modified Golgi-Cox method. J Neurosci Methods 256:141–150. https://doi.org/10.1016/j.jneumeth.2015.08.029
Soares LM, De Vry J, Steinbusch HWM, Milani H, Prickaerts J, Weffort de Oliveira RM (2016) Rolipram improves cognition, reduces anxiety- and despair-like behaviors and impacts hippocampal neuroplasticity after transient global cerebral ischemia. Neuroscience 326:69–83. https://doi.org/10.1016/j.neuroscience.2016.03.062
Block F (1999) Global ischemia and behavioural deficits. Prog Neurobiol 58(3):279–295. https://doi.org/10.1016/s0301-0082(98)00085-9
Erfani S, Moghimi A, Aboutaleb N, Khaksari M (2018) Nesfatin-1 Improve Spatial Memory Impairment Following Transient Global Cerebral Ischemia/Reperfusion via Inhibiting Microglial and Caspase-3 Activation. J Mol Neurosci 65(3):377–384. https://doi.org/10.1007/s12031-018-1105-3
Montes P, Vigueras-Villaseñor RM, Rojas-Castañeda JC, Monfil T, Cervantes M, Moralí G (2019) Progesterone treatment in rats after severe global cerebral ischemia promotes hippocampal dentate gyrus neurogenesis and functional recovery. Neurol Res 41(5):429–436. https://doi.org/10.1080/01616412.2019.1576356
Zhang B, Zhong Q, Chen X, Wu X, Sha R, Song G, Zhang C, Chen X (2020) Neuroprotective effects of celastrol on transient global cerebral ischemia rats via regulating HMGB1/NF-κB signaling pathway. Front Neurosci 14:847. https://doi.org/10.3389/fnins.2020.00847
Paganelli RA, Benetoli A, Milani H (2006) Sustained neuroprotection and facilitation of behavioral recovery by the Ginkgo biloba extract, EGb 761, after transient forebrain ischemia in rats. Behav Brain Res 174(1):70–77. https://doi.org/10.1016/j.bbr.2006.07.005
Baccarin CC, Godinho J, de Oliveira RMW, Matsushita M, Gohara AK, Cardozo-Filho L, Lima JC, Previdelli IS, Melo SR, Ribeiro MHDM, Milani H (2016) Postischemic fish oil treatment restores long-term retrograde memory and dendritic density: an analysis of the time window of efficacy. Behav Brain Res 311:425–439. https://doi.org/10.1016/j.bbr.2016.05.047
Bonato JM, Meyer E, de Mendonça PSB, Milani H, Prickaerts J, Weffort de Oliveira RM (2021) Roflumilast protects against spatial memory impairments and exerts anti-inflammatory effects after transient global cerebral ischemia. Eur J Neurosci 53(4):1171–1188. https://doi.org/10.1111/ejn.15089
Santiago AN, Mori MA, Guimarães FS, Milani H, Weffort de Oliveira RM (2019) Effects of cannabidiol on diabetes outcomes and chronic cerebral hypoperfusion comorbidities in middle-aged rats. Neurotox Res 35(2):463–474. https://doi.org/10.1007/s12640-018-9972-5
Fagherazzi EV, Garcia VA, Maurmann N, Bervanger T, Halmenschlager LH, Busato SB, Hallak JE, Zuardi AW, Crippa JA, Schröder N (2012) Memory rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology (Berl) 219(4):1133–1140. https://doi.org/10.1007/s00213-011-2449-3
Lee JLC, Bertoglio LJ, Guimarães FS, Stevenson CW (2017) Cannabidiol regulation of emotion and emotional memory processing: relevance for treating anxiety-related and substance abuse disorders. Br J Pharmacol 174(19):3242–3256. https://doi.org/10.1111/bph.13724
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297(5868):681–683. https://doi.org/10.1038/297681a0
Nunn JA, LePeillet E, Netto CA, Hodges H, Gray JA, Meldrum BS (1994) Global ischaemia: hippocampal pathology and spatial deficits in the water maze. Behav Brain Res 62(1):41–54. https://doi.org/10.1016/0166-4328(94)90036-1
Hartman RE, Lee JM, Zipfel GJ, Wozniak DF (2005) Characterizing learning deficits and hippocampal neuron loss following transient global cerebral ischemia in rats. Brain Res 1043(1–2):48–56. https://doi.org/10.1016/j.brainres.2005.02.030
Braida D, Pegorini S, Arcidiacono MV, Consalez GG, Croci L, Sala M (2003) Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion, and neuronal injury in gerbils. Neurosci Lett 346(1–2):61–64. https://doi.org/10.1016/s0304-3940(03)00569-x
Bacarin CC, Mori MA, Ferreira EDF, Romanini CV, de Oliveira RMW, Milani H (2013) Fish oil provides robust and sustained memory recovery after cerebral ischemia: influence of treatment regimen. Physiol Behav 119:61–71. https://doi.org/10.1016/j.physbeh.2013.06.001
Bacarin CC, Godinho J, de Oliveira RMW, Matsushita M, Gohara AK, Cardozo-Filho L, Lima JC, Previdelli IS, Melo SR, Ribeiro MHDM, Milani H (2016) Postischemic fish oil treatment restores long-term retrograde memory and dendritic density: An analysis of the time window of efficacy. Behav Brain Res 311:425–439. https://doi.org/10.1016/j.bbr.2016.05.047
de Oliveira JN, Reis LO, Ferreira EDF, Godinho J, Bacarin CC, Soares LM, de Oliveira RMW, Milani H (2017) Postischemic fish oil treatment confers task-dependent memory recovery. Physiol Behav 177:196–207. https://doi.org/10.1016/j.physbeh.2017.05.009
Aronowski J, Samways E, Strong R, Rhoades HM, Grotta JC (1996) An alternative method for the quantitation of neuronal damage after experimental middle cerebral artery occlusion in rats: analysis of behavioral deficits. J Cereb Blood Flow Metab 16(4):705–713. https://doi.org/10.1097/00004647-199607000-00022
Bachevalier J, Meunier M (1996) Cerebral ischemia: are the memory deficits associated with hippocampal cell loss? Hippocampus 6(5):553–560. https://doi.org/10.1002/(SICI)1098-1063(1996)6:5%3c553::AID-HIPO8%3e3.0.CO;2-J
Kawai T, Takagi N, Miyake-Takagi K, Okuyama N, Mochizuki N, Takeo S (2004) Characterization of BrdU-positive neurons induced by transient global ischemia in adult hippocampus. J Cereb Blood Flow Metab 24(5):548–555. https://doi.org/10.1097/00004647-200405000-00009
Soares LM, Schiavon AP, Milani H, de Oliveira RM (2013) Cognitive impairment and persistent anxiety-related responses following bilateral common carotid artery occlusion in mice. Behav Brain Res 249:28–37. https://doi.org/10.1016/j.bbr.2013.04.010
García-Chávez D, González-Burgos I, Letechipía-Vallejo G, López-Loeza E, Moralí G, Cervantes M (2008) Long-term evaluation of cytoarchitectonic characteristics of prefrontal cortex pyramidal neurons, following global cerebral ischemia and neuroprotective melatonin treatment, in rats. Neurosci Lett 448(1):148–152. https://doi.org/10.1016/j.neulet.2008.10.043
Murphy TH, Corbett D (2009) Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 10(12):861–872. https://doi.org/10.1038/nrn2735
Begni V, Riva MA, Cattaneo A (2017) Cellular and molecular mechanisms of the brain-derived neurotrophic factor in physiological and pathological conditions. Clin Sci (Lond) 131(2):123–138. https://doi.org/10.1042/CS20160009
Schäbitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG (2007) Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke 38(7):2165–2172. https://doi.org/10.1161/STROKEAHA.106.477331
Espinera AR, Ogle ME, Gu X, Wei L (2013) Citalopram enhances neurovascular regeneration and sensorimotor functional recovery after ischemic stroke in mice. Neuroscience 247:1–11. https://doi.org/10.1016/j.neuroscience.2013.04.011
Aguiar RP, Soares LM, Meyer E, da Silveira FC, Milani H, Newman-Tancredi A, Varney M, Prickaerts J, Oliveira RMW (2020) Activation of 5-HT1A postsynaptic receptors by NLX-101 results in functional recovery and an increase in neuroplasticity in mice with brain ischemia. Prog Neuropsychopharmacol Biol Psychiatry 99:109832. https://doi.org/10.1016/j.pnpbp.2019.109832
Cheng CY, Kao ST (2020) Lee YC (2020) Angelica sinensis extract protects against ischemia-reperfusion injury in the hippocampus by activating p38 MAPK-mediated p90RSK/p-Bad and p90RSK/CREB/BDNF signaling after transient global cerebral ischemia in rats. J Ethnopharmacol 24(252):112612. https://doi.org/10.1016/j.jep.2020.112612
Wang W, Liu X, Yang Z, Shen H, Liu L, Yu Y, Zhang T (2020) Levodopa improves cognitive function and the deficits of structural synaptic plasticity in hippocampus induced by global cerebral ischemia/reperfusion injury in rats. Front Neurosci. 30(4):586321. https://doi.org/10.3389/fnins.2020.586321
de la Tremblaye PB, Benoit SM, Schock S, Plamondon H (2017) CRHR1 exacerbates the glial inflammatory response and alters BDNF/TrkB/pCREB signaling in a rat model of global cerebral ischemia: implications for neuroprotection and cognitive recovery. Prog Neuropsychopharmacol Biol Psychiatry 3:234–248. https://doi.org/10.1016/j.pnpbp.2017.06.021
Kapoor M, Sharma S, Sandhir R, Nehru B (2019) Temporal changes in physiological and molecular markers in various brain regions following transient global ischemia in rats. Mol Biol Rep 46(6):6215–6230. https://doi.org/10.1007/s11033-019-05060-7
Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, Fujiwara M (2005) Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke 36(5):1077–1082. https://doi.org/10.1161/01.STR.0000163083.59201.34
Sales AJ, Fogaça MV, Sartim AG, Pereira VS, Wegener G, Guimarães FS, Joca SRL (2019) Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol Neurobiol 56(2):1070–1081. https://doi.org/10.1007/s12035-018-1143-4
Sadigh-Eteghad S, Geranmayeh MH, Majdi A, Salehpour F, Mahmoudi J, Farhoudi M (2018) Intranasal cerebrolysin improves cognitive function and structural synaptic plasticity in photothrombotic mouse model of medial prefrontal cortex ischemia. Neuropeptides 71:61–69. https://doi.org/10.1016/j.npep.2018.07.002
Tarsa L, Goda Y (2002) Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A 99(2):1012–1016. https://doi.org/10.1073/pnas.022575999
Luo J, Zhang L, Ning N, Jiang H, Yu SY (2013) Neotrofin reverses the effects of chronic unpredictable mild stress on behavior via regulating BDNF, PSD-95 and synaptophysin expression in rat. Behav Brain Res 253:48–53. https://doi.org/10.1016/j.bbr.2013.07.014
Ishimaru H, Casamenti F, Uéda K, Maruyama Y, Pepeu G (2001) Changes in presynaptic proteins, SNAP-25 and synaptophysin, in the hippocampal CA1 area in ischemic gerbils. Brain Res 903(1–2):94–101. https://doi.org/10.1016/s0006-8993(01)02439-8
Zhao Y, Wang J, Liu C, Jiang C, Zhao C, Zhu Z (2011) Progesterone influences postischemic synaptogenesis in the CA1 region of the hippocampus in rats. Synapse 65(9):880–891. https://doi.org/10.1002/syn.20915
Yan BC, Park JH, Ahn JH, Lee JC, Won MH, Kang IJ (2013) Postsynaptic density protein (PSD)-95 expression is markedly decreased in the hippocampal CA1 region after experimental ischemia-reperfusion injury. J Neurol Sci 330(1–2):111–116. https://doi.org/10.1016/j.jns.2013.04.023
da Silva VK, de Freitas BS, da Silva DA, Nery LR, Falavigna L, Ferreira RD, Bogo MR, Hallak JE, Zuardi AW, Crippa JA, Schröder N (2014) Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection. Mol Neurobiol 49(1):222–233. https://doi.org/10.1007/s12035-013-8514-7
González-Burgos I, Letechipía-Vallejo G, López-Loeza E, Moralí G, Cervantes M (2007) Long-term study of dendritic spines from hippocampal CA1 pyramidal cells, after neuroprotective melatonin treatment following global cerebral ischemia in rats. Neurosci Lett 423(2):162–166. https://doi.org/10.1016/j.neulet.2007.06.050
Hasbani MJ, Schlief ML, Fisher DA, Goldberg MP (2001) Dendritic spines lost during glutamate receptor activation reemerge at original sites of synaptic contact. J Neurosci 21(7):2393–2403. https://doi.org/10.1523/JNEUROSCI.21-07-02393.2001
Shih PC, Yang YR, Wang RY (2013) Effects of exercise intensity on spatial memory performance and hippocampal synaptic plasticity in transient brain ischemic rats. PLoS One 8(10):e78163. https://doi.org/10.1371/journal.pone.0078163
Moralí G, Montes P, González-Burgos I, Velázquez-Zamora DA, Cervantes M (2012) Cytoarchitectural characteristics of hippocampal CA1 pyramidal neurons of rats, four months after global cerebral ischemia and progesterone treatment. Restor Neurol Neurosci 30(1):1–8. https://doi.org/10.3233/RNN-2011-0605
Kocsis K, Knapp L, Gellért L, Oláh G, Kis Z, Takakuwa H, Iwamori N, Ono E, Toldi J, Farkas T (2014) Acetyl-L-carnitine normalizes the impaired long-term potentiation and spine density in a rat model of global ischemia. Neuroscience 269:265–672. https://doi.org/10.1016/j.neuroscience.2014.03.055
Mori MA, Meyer E, da Silva FF, Milani H, Guimarães FS, Oliveira RMW (2021) Differential contribution of CB1, CB2, 5-HT1A, and PPAR-γ receptors to cannabidiol effects on ischemia-induced emotional and cognitive impairments. Eur J Neurosci. https://doi.org/10.1111/ejn.15134
Fernández-Ruiz J, Sagredo O, Pazos MR, García C, Pertwee R, Mechoulam R, Martínez-Orgado J (2013) Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol 75(2):323–333. https://doi.org/10.1111/j.1365-2125.2012.04341.x
Campos AC, Fogaça MV, Sonego AB, Guimarães FS (2016) Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res 112:119–127. https://doi.org/10.1016/j.phrs.2016.01.033
Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34(5):605–613
Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V (2001) Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134(4):845–852. https://doi.org/10.1038/sj.bjp.0704327
Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG (2007) Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol 150(5):613–623. https://doi.org/10.1038/sj.bjp.0707133
Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, Cipriano M, Carratù MR, Iuvone T, Steardo L (2011) Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One 6(12):e28668. https://doi.org/10.1371/journal.pone.0028668
Brown KJ, Laun AS, Song ZH (2017) Cannabidiol, a novel inverse agonist for GPR12. Biochem Biophys Res Commun 493(1):451–454. https://doi.org/10.1016/j.bbrc.2017.09.001
Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Vitoretti LB, Mariano-Souza DP, Quinteiro-Filho WM, Akamine AT, Almeida VI, Quevedo J, Dal-Pizzol F, Hallak JE, Zuardi AW, Crippa JA, Palermo-Neto J (2012) Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: role for the adenosine A(2A) receptor. Eur J Pharmacol 678(1–3):78–85. https://doi.org/10.1016/j.ejphar.2011.12.043
Valvassori SS, Bavaresco DV, Scaini G, Varela RB, Streck EL, Chagas MH, Hallak JE, Zuardi AW, Crippa JA, Quevedo J (2013) Acute and chronic administration of cannabidiol increases mitochondrial complex and creatine kinase activity in the rat brain. Braz J Psychiatry 35(4):380–386. https://doi.org/10.1590/1516-4446-2012-0886
Acknowledgements
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Universidade Estadual de Maringá and FAPESP (2017/24304-0), São Paulo, Brazil. The authors thank ADCA – Indústria e Comércio de Material Cirúrgico for kindly donating the aneurysm clips and Marco Alberto Trombelli for his technical support.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Universidade Estadual de Maringá, Paraná, Brazil and FAPESP (2017/24304–0), São Paulo, Brazil.
Author information
Authors and Affiliations
Contributions
EM and RMWO conceived and designed the experiments with inputs from ACC. EM performed behavioral tests, western blot, and Golgi analysis. She wrote the first draft of the manuscript. EM and JMB conducted the animals’ surgeries. BAM performed the immunohistochemistry. MAM performed data analysis. FSG and HM helped with statistical analysis and data interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Research Involving Human Participants and/and or Animals
The local Ethics Committee on Animal Experimentation of the State University of Maringá approved the experimental procedures in accordance with the guidelines of the U.S. National Institutes of Health and Brazilian College for Animal Experimentation (animal license number: CEUA 1555230316).
Ethics Approval and Consent to Participate
This study was carried out at the State University of Maringá in strict accordance with the Brazilian College of Animal Experimentation (COBEA) recommendations. Animal experiments were approved by the local Ethics Committee on Animal Experimentation of the State University of Maringá (animal license number: CEUA 1555230316).
Consent for Publication
All of the co-authors approved the final version of the manuscript and agreed to submit it to Molecular Neurobiology.
Competing Interests
FSG is a co-inventor (Mechoulam R, JC, Guimaraes FS, AZ, JH, Breuer A) of the patent “Fluorinated CBD compounds, compositions, and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023” Def. US No. Reg. 62193296; 29/07/2015; INPI on 19/08/2015 (BR1120150164927). The University of São Paulo has licensed the patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1). The University of São Paulo has an agreement with Prati-Donaduzzi (Toledo, Brazil) to “develop a pharmaceutical product containing synthetic cannabidiol and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson’s disease, and anxiety disorders.”
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meyer, E., Bonato, J.M., Mori, M.A. et al. Cannabidiol Confers Neuroprotection in Rats in a Model of Transient Global Cerebral Ischemia: Impact of Hippocampal Synaptic Neuroplasticity. Mol Neurobiol 58, 5338–5355 (2021). https://doi.org/10.1007/s12035-021-02479-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02479-7