Abstract
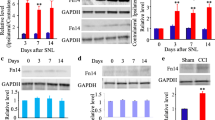
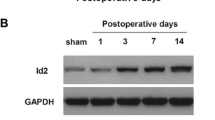
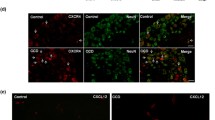
Neuropathic pain, resulting from the pathological changes of the somatosensory nervous system, remains a severe public health problem worldwide. The effect of treatment targeting neuropathic pain is very limited, as the underlying mechanism of neuropathic pain is largely unknown. In this study, we demonstrated that the expression level of brain-derived neurotrophic factor (BDNF) was remarkably and time-dependently increased in dorsal root ganglion (DRG) neurons. DRG microinjection of BDNF siRNA in DRG ameliorated chronic constriction injury (CCI) induced mechanical, thermal, and cold nociceptive hypersensitivities. Overexpressing BDNF through microinjection of the AAV5-BDNF in DRG caused enhanced responses to basal mechanical, thermal, and cold stimuli in mice exposed to CCI. Mechanically, the P2X7 promoter activity was enhanced by CCI-induced increase of DRG BDNF protein and was involved in the CCI-induced upregulation of DRG P2X7 protein. The overexpression of BDNF also increased P2X7 expression in DRG neurons, which was validated in in vivo and in vitro experiments. BDNF may exert crucial effect via transcriptionally activating the P2X7 gene in primary sensory neurons, since P2X7 acts as a role of endogenous agitator in neuropathic pain and BDNF largely co-expresses with P2X7 in DRG neurons. Therefore, our data provide evidence that BDNF may be a promising therapeutic target for neuropathic pain.






Similar content being viewed by others
Data Availability
Data will be made available on reasonable request.
References
Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, Benoliel R, Cohen M et al (2019) The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 160(1):53–59
van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N (2014) Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 155(4):654–662
Cohen SP, Mao J (2014) Neuropathic pain: mechanisms and their clinical implications. BMJ 348:f7656
St JSE (2018) Advances in understanding nociception and neuropathic pain. J Neurol 265(2):231–238
Thompson SW, Bennett DL, Kerr BJ, Bradbury EJ, McMahon SB (1999) Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci U S A 96(14):7714–7718
McMahon SB, Cafferty WB (2004) Neurotrophic influences on neuropathic pain. Novartis Found Symp 261: 68–92; discussion 92–102, 149–54
Pezet S, McMahon SB (2006) Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29:507–538
Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW et al (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438(7070):1017–1021
Ha SO, Kim JK, Hong HS, Kim DS, Cho HJ (2001) Expression of brain-derived neurotrophic factor in rat dorsal root ganglia, spinal cord and gracile nuclei in experimental models of neuropathic pain. Neuroscience 107(2):301–309
Li YX, Xu Y, Ju D, Lester HA, Davidson N, Schuman EM (1998) Expression of a dominant negative TrkB receptor, T1, reveals a requirement for presynaptic signaling in BDNF-induced synaptic potentiation in cultured hippocampal neurons. Proc Natl Acad Sci U S A 95(18):10884–10889
Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Narita M, Yamaguchi T, Tamaki H et al (2005) Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem 93(3):584–594
MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M (2001) Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci 115(5):1145–1153
Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB et al (1999) Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci 19(12):5138–5148
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33(1):87–107
Zhang J, Liang L, Miao X, Wu S, Cao J, Tao B, Mao Q, Mo K et al (2016) Contribution of the suppressor of variegation 3–9 homolog 1 in dorsal root ganglia and spinal cord dorsal horn to nerve injury-induced nociceptive hypersensitivity. Anesthesiology 125(4):765–778
Xu JT, Zhao J-Y, Zhao X, Ligons D, Tiwari V, Atianjoh FE, Lee C-Y, Liang L et al (2014) Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest 124(2):592–603
Li Z, Gu X, Sun L, Wu S, Liang L, Cao J, Lutz BM, Bekker A et al (2015) Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma. Pain 156(4):711–721
Li J, Li D, Zhou H, Wu G, He Z, Liao W, Li Y, Zhi Y (2020) MicroRNA-338–5p alleviates neuronal apoptosis via directly targeting BCL2L11 in APP/PS1 mice. Aging (Albany NY) 12(20):20728–20742
Li J, Guo M, Liu Y, Wu G, Miao L, Zhang J, Zuo Z, Li Y (2019) Both GSK-3β/CRMP2 and CDK5/CRMP2 pathways participate in the protection of dexmedetomidine against propofol-induced learning and memory impairment in neonatal rats. Toxicol Sci kfz135
Li J, Wu G, Song W, Liu Y, Han Z, Shen Z, Li Y (2020) Prophylactic melatonin treatment ameliorated propofol-induced cognitive dysfunction in aged rats. Neurotox Res 39(2):227–239
Tao YX, Rumbaugh G, Wang G-D, Petralia RS, Zhao C, Kauer FW, Tao F, Zhuo M et al (2003) Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci 23(17):6703–6712
Zhao X, Tang Z, Zhang H, Atianjoh FE, Zhao J-Y, Liang L, Wang W, Guan X et al (2013) A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci 16(8):1024–1031
Wu G, Zhou H, Li D, Zhi Y, Liu Y, Li J, Wang F (2020) LncRNA DANCR upregulation induced by TUFT1 promotes malignant progression in triple negative breast cancer via miR-874-3p-SOX2 axis. Exp Cell Res 396(2):112331
Xie J, Liu S, Wu B, Li G, Rao S, Zou L, Yi Z, Zhang C et al (2017) The protective effect of resveratrol in the transmission of neuropathic pain mediated by the P2X(7) receptor in the dorsal root ganglia. Neurochem Int 103:24–35
Ge H, Guan S, Shen Y, Sun M, Hao Y, He L, Liu L, Yin C et al (2019) Dihydromyricetin affects BDNF levels in the nervous system in rats with comorbid diabetic neuropathic pain and depression. Sci Rep 9(1):14619
Nijs J, Meeus M, Versijpt J, Moens M, Bos I, Knaepen K, Meeusen R (2015) Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: a new therapeutic target? Expert Opin Ther Targets 19(4):565–576
Zhang H, Qian Y-L, Li C, Liu D, Wang L, Wang X-Y, Liu M-J, Liu H et al (2017) Brain-derived neurotrophic factor in the mesolimbic reward circuitry mediates nociception in chronic neuropathic pain. Biol Psychiatry 82(8):608–618
Skaper SD, Debetto P, Giusti P (2010) The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J 24(2):337–345
Burnstock G, Krügel U, Abbracchio MP, Illes P (2011) Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol 95(2):229–274
Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS (2011) Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 63(3):641–683
Yan Z, Khadra A, Sherman A, Stojilkovic SS (2011) Calcium-dependent block of P2X7 receptor channel function is allosteric. J Gen Physiol 138(4):437–452
Broom DC, Matson DJ, Bradshaw E, Buck ME, Meade R, Coombs S, Matchett M, Ford KK et al (2008) Characterization of N-(adamantan-1-ylmethyl)-5-[(3R-amino-pyrrolidin-1-yl)methyl]-2-chloro-benzamide, a P2X7 antagonist in animal models of pain and inflammation. J Pharmacol Exp Ther 327(3):620–633
Perez-Medrano A, Donnelly-Roberts DL, Honore P, Hsieh GC, Namovic MT, Peddi S, Shuai Q, Wang Y et al (2009) Discovery and biological evaluation of novel cyanoguanidine P2X(7) antagonists with analgesic activity in a rat model of neuropathic pain. J Med Chem 52(10):3366–3376
Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M et al (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114(3):386–396
Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q et al (2004) P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med 10(8):821–827
Funding
This work was supported by the Natural Science Foundation of Guangdong Province, China (No.2016A030313251, No.2018A0303130272); and the Science and Technology Planning Project of Guangzhou, China (No. 201707010207).
Author information
Authors and Affiliations
Contributions
Yi Wu, Zhiwen Shen, and Hui Xu: investigation, methodology, writing-original draft preparation. Kun Zhang: visualization, software. Mingyan Guo: conceptualization, data curation. Fei Wang and Junhua Li: writing-reviewing and editing.
Corresponding authors
Ethics declarations
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Y., Shen, Z., Xu, H. et al. BDNF Participates in Chronic Constriction Injury-Induced Neuropathic Pain via Transcriptionally Activating P2X7 in Primary Sensory Neurons. Mol Neurobiol 58, 4226–4236 (2021). https://doi.org/10.1007/s12035-021-02410-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02410-0