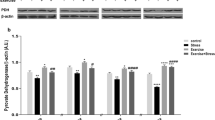
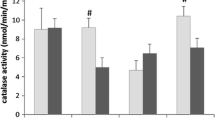
Abstract
Oxidative stress associated with chronic cerebral hypoperfusion is one of the fundamental factors leading to neurodegenerative diseases. To prevent oxidative stress, physical activity is effective. Physical exercise enables development of rehabilitation techniques that can progressively increase patients’ stress resistance. We determined the oxidative stress dynamics in experimental hypoperfusion and modeled rehabilitation measures, comparing sex and stress resistance levels. The experiment was performed on 240 Wistar rats of both sexes over a period of 90 days. Based on behavioral test results obtained using the open field test, the rats were divided into active animals with predicted higher stress resistance (HSR) and passive animals with predicted lower stress resistance (LSR). TBA (thiobarbituric acid) plasma concentration of the active products (malondialdehyde—MDA), blood plasma (NO-X) concentration, and l-citrulline (LC) concentration were determined spectrophotometrically at the corresponding wave length (nm). The intensity of oxidative stress was evaluated using the chemoluminscent method to determine the blood plasma antioxidant activity on the BCL-07 biochemoluminometer. This study revealed two stages of oxidative stress: a less pronounced phase covering the first days after surgery and a main one, which starts from the month after the operation to 3 months. Female sex and a high initial level of stress resistance reduced the severity of oxidative stress. Physical activity commencing a week after the surgery resulted in “reloading” the adaptive mechanisms and slowed the onset of the main stage, leading to a decrease in the free-radical process in all studied subgroups and the greater blood plasma (NO)-X decrease in the male animals. Future neuropharmacological intervention most likely will be able to determine the pathophysiology mechanism of chronic brain hypoperfusion and potentially extending adaptive responses.

Graphical abstract





Similar content being viewed by others
References
Anderson RM, Johnson SB, Lingg RT, Hinz DC, Romig-Martin SA, Radley JJ (2019) Evidence for similar prefrontal structural and functional alterations in male and female rats following chronic stress or glucocorticoid exposure. Cereb Cortex 30:1–18. https://doi.org/10.1093/cercor/bhz092
Banqueri M, Mendez M, Gomez-Lazaro E, Arias JL (2019) Early life stress by repeated maternal separation induces long-term neuroinflammatory response in glial cells of male rats. Stress 22(5):563–570. https://doi.org/10.1080/10253890.2019.1604666
Benatti C, Radighieri G, Alboni S, Blom JMC, Brunello N, Tascedda F (2019) Modulation of neuroplasticity-related targets following stress-induced acute escape deficit. Behav Brain Res 364:140–148. https://doi.org/10.1016/j.bbr.2019.02.023
Issam N, Raffaello S, Dafne S, Luigi C, Abdelkrim T (2019) A simple approach to studying cerebral blood flow during psychological stress. Naunyn Schmiedeberg's Arch Pharmacol 392(4):505–509. https://doi.org/10.1007/s00210-019-01638-x
Naskar S, Chattarji S (2019) Stress elicits contrasting effects on the structure and number of astrocytes in the amygdala versus hippocampus. eNeuro 6(1). https://doi.org/10.1523/eneuro.0338-18.2019 ENEURO.0338, ENEU18.2019
Moench KM, Breach MR, Wellman CL (2019) Chronic stress produces enduring sex- and region-specific alterations in novel stress-induced c-Fos expression. Neurobiol Stress 10:100147. https://doi.org/10.1016/j.ynstr.2019.100147
Akimoto H, Oshima S, Sugiyama T, Negishi A, Nemoto T, Kobayashi D (2019) Changes in brain metabolites related to stress resilience: metabolomic analysis of the hippocampus in a rat model of depression. Behav Brain Res 359:342–352. https://doi.org/10.1016/j.bbr.2018.11.017
Wislowska-Stanek A, Plaznik A, Kolosowska K, Skorzewska A, Turzynska D, Liguz-Lecznar M, Krzascik P, Gryz M et al (2019) Differences in the dopaminergic reward system in rats that passively and actively behave in the Porsolt test. Behav Brain Res 359:181–189. https://doi.org/10.1016/j.bbr.2018.10.027
Jianguo L, Xueyang J, Cui W, Changxin W, Xuemei Q (2019) Altered gut metabolome contributes to depression-like behaviors in rats exposed to chronic unpredictable mild stress. Transl Psychiatry 9(1):40. https://doi.org/10.1038/s41398-019-0391-z
Dmitrieva NV, Koplik EV, Ioffe ML, Voronov EB, Parfent'ev NA (1990) low-molecular peptides in food as factors altering the resistance of rats to emotional stress. Patol Fiziol Eksp Ter (4):11–13
Kent DM, Ruthazer R, Decker C, Jones PG, Saver JL, Bluhmki E, Spertus JA (2015) Development and validation of a simplified stroke-thrombolytic predictive instrument. Neurology 85(11):942–949. https://doi.org/10.1212/wnl.0000000000001925
Ghonimi NAM, Mahdy ME, Abdel Salam OA (2019) Total antioxidant capacity predicts outcome in acute ischemic stroke subtypes in Egyptian patients. J Stroke Cerebrovasc Dis 28(7):1911–1917. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.03.053
Moreira PI, Siedlak SL, Aliev G, Zhu X, Cash AD, Smith MA, Perry G (2005) Oxidative stress mechanisms and potential therapeutics in Alzheimer disease. J Neural Transm (Vienna) 112(7):921–932. https://doi.org/10.1007/s00702-004-0242-8
Martinez Leo EE, Segura Campos MR (2019) Systemic oxidative stress: a key point in neurodegeneration - a review. J Nutr Health Aging 23(8):694–699. https://doi.org/10.1007/s12603-019-1240-8
Milaeva ER, Gerasimova OA, Jingwei Z, Shpakovsky DB, Syrbu SA, Semeykin AS, Koifman OI, Kireeva EG et al (2008) Synthesis and antioxidative activity of metalloporphyrins bearing 2,6-di-tert-butylphenol pendants. J Inorg Biochem 102(5–6):1348–1358. https://doi.org/10.1016/j.jinorgbio.2008.01.022
Perlovich GL, Proshin AN, Volkova TV, Petrova LN, Bachurin SO (2012) Novel 1,2,4-thiadiazole derivatives as potent neuroprotectors: approach to creation of bioavailable drugs. Mol Pharm 9(8):2156–2167. https://doi.org/10.1021/mp300011r
Shevtsova EF, Vinogradova DV, Kireeva EG, Reddy VP, Aliev G, Bachurin SO (2014) Dimebon attenuates the Abeta-induced mitochondrial permeabilization. Curr Alzheimer Res 11(5):422–429. https://doi.org/10.2174/1567205011666140505094808
Bachurin SO, Shevtsova EF, Makhaeva GF, Grigoriev VV, Boltneva NP, Kovaleva NV, Lushchekina SV, Shevtsov PN et al (2017) Novel conjugates of aminoadamantanes with carbazole derivatives as potential multitarget agents for AD treatment. Sci Rep 7:45627. https://doi.org/10.1038/srep45627
Neganova ME, Klochkov SG, Afanasieva SV, Serkova TP, Chudinova ES, Bachurin SO, Reddy VP, Aliev G et al (2016) Neuroprotective effects of the securinine-analogues: Identification of allomargaritarine as a lead compound. CNS Neurol Disord Drug Targets 15(1):102–107. https://doi.org/10.2174/1871527314666150821111812
Liu Y, Yan T, Chu JM, Chen Y, Dunnett S, Ho YS, Wong GT, Chang RC (2019) The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab Investig 99(7):943–957. https://doi.org/10.1038/s41374-019-0232-y
Uijen IL, Aaronson JA, Karssemeijer EGA, Olde Rikkert MGM, Kessels RPC (2020) Individual differences in the effects of physical activity on cognitive function in people with mild to moderate dementia. J Alzheimers Dis 74(2):435–439. https://doi.org/10.3233/jad-190606
Fayyaz M, Jaffery SS, Anwer F, Zil EAA, Anjum I (2018) The effect of physical activity in Parkinson’s disease: a mini-review. Cureus 10(7):e2995. https://doi.org/10.7759/cureus.2995
Pang TY, Hannan AJ (2013) Enhancement of cognitive function in models of brain disease through environmental enrichment and physical activity. Neuropharmacology 64:515–528. https://doi.org/10.1016/j.neuropharm.2012.06.029
Ahlskog JE (2011) Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 77(3):288–294. https://doi.org/10.1212/WNL.0b013e318225ab66
Shen H, Tong L, Balazs R, Cotman CW (2001) Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience 107(2):219–229. https://doi.org/10.1016/s0306-4522(01)00315-3
Mota MP, Dos Santos ZA, Soares JFP, de Fatima PA, Joao PV, O'Neil Gaivao I, Oliveira MM (2019) Intervention with a combined physical exercise training to reduce oxidative stress of women over 40years of age. Exp Gerontol 123:1–9. https://doi.org/10.1016/j.exger.2019.05.002
Arisha AH, Moustafa A (2019) Potential inhibitory effect of swimming exercise on the Kisspeptin-GnRH signaling pathway in male rats. Theriogenology 133:87–96. https://doi.org/10.1016/j.theriogenology.2019.04.035
Morgan JA, Corrigan F, Baune BT (2015) Effects of physical exercise on central nervous system functions: a review of brain region specific adaptations. J Mol Psychiatry 3(1):3. https://doi.org/10.1186/s40303-015-0010-8
Radak Z, Sasvari M, Nyakas C, Kaneko T, Tahara S, Ohno H, Goto S (2001) Single bout of exercise eliminates the immobilization-induced oxidative stress in rat brain. Neurochem Int 39(1):33–38. https://doi.org/10.1016/s0197-0186(01)00003-1
Rossi Dare L, Garcia A, Alves N, Ventura Dias D, de Souza MA, Mello-Carpes PB (2019) Physical and cognitive training are able to prevent recognition memory deficits related to amyloid beta neurotoxicity. Behav Brain Res 365:190–197. https://doi.org/10.1016/j.bbr.2019.03.007
Quines CB, Jardim NS, Araujo PCO, Cechella JL, Prado VC, Nogueira CW (2019) Resistance training restores metabolic alterations induced by monosodium glutamate in a sex-dependent manner in male and female rats. J Cell Biochem 120(8):13426–13440. https://doi.org/10.1002/jcb.28617
Robbins JM, Herzig M, Morningstar J, Sarzynski MA, Cruz DE, Wang TJ, Gao Y, Wilson JG et al (2019) Association of dimethylguanidino valeric acid with partial resistance to metabolic health benefits of regular exercise. JAMA Cardiol 4(7):636–643. https://doi.org/10.1001/jamacardio.2019.1573
Tanner MK, Fallon IP, Baratta MV, Greenwood BN (2019) Voluntary exercise enables stress resistance in females. Behav Brain Res 369:111923. https://doi.org/10.1016/j.bbr.2019.111923
Koplik EV, Salieva RM, Gorbunova AV (1995) The open-field test as a prognostic criterion of resistance to emotional stress in Wistar rats. Zh Vyssh Nerv Deiat Im I P Pavlova 45(4):775–781
Xiao-Li W, Liang L, Hui Z, Xing W, Zhi-Yong T, Hui X, Yuan-Yuan W, Xiao-di Y et al (2016) Effect of exogenous nitric oxide on antioxidants from mice infected with Trichinella spiralis. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 29(1):48–52. https://doi.org/10.16250/j.32.1374.2016196
Ozcelik O, Algul S (2017) Nitric oxide levels in response to the patients with different stage of diabetes. Cell Mol Biol (Noisy-le-grand) 63(1):49–52. https://doi.org/10.14715/cmb/2017.63.1.10
Savel'ev SA, Repkina NS (2005) A sensitive technique for determining citrulline for the purpose of in vivo monitoring of the nitrogen oxide production in the CHS. Ross Fiziol Zh Im I M Sechenova 91(5):587–591
Kong Y, Trabucco SE, Zhang H (2014) Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Interdiscip Top Gerontol 39:86–107. https://doi.org/10.1159/000358901
Gliemann L, Nyberg M, Hellsten Y (2014) Nitric oxide and reactive oxygen species in limb vascular function: what is the effect of physical activity? Free Radic Res 48(1):71–83. https://doi.org/10.3109/10715762.2013.835045
Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jimenez MC, Kittner SJ, Madsen TE et al (2018) Sex differences in stroke: challenges and opportunities. J Cereb Blood Flow Metab 38(12):2179–2191. https://doi.org/10.1177/0271678x18793324
Charriaut-Marlangue C, Besson VC, Baud O (2017) Sexually dimorphic outcomes after neonatal stroke and hypoxia-ischemia. Int J Mol Sci 19(1). https://doi.org/10.3390/ijms19010061
Horecky J, Gvozdjakova A, Kucharska J, Obrenovich ME, Palacios HH, Li Y, Vancova O, Aliev G (2011) Effects of coenzyme Q and creatine supplementation on brain energy metabolism in rats exposed to chronic cerebral hypoperfusion. Curr Alzheimer Res 8(8):868–875. https://doi.org/10.2174/156720511798192727
Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F (2018) Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol 14:450–464. https://doi.org/10.1016/j.redox.2017.10.014
Shenk JC, Liu J, Fischbach K, Xu K, Puchowicz M, Obrenovich ME, Gasimov E, Alvarez LM et al (2009) The effect of acetyl-L-carnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer’s disease. J Neurol Sci 283(1–2):199–206. https://doi.org/10.1016/j.jns.2009.03.002
Yamamoto T, Ohkuwa T, Itoh H, Sato Y, Naoi M (2002) Effect of gender differences and voluntary exercise on antioxidant capacity in rats. Comp Biochem Physiol C Toxicol Pharmacol 132(4):437–444. https://doi.org/10.1016/s1532-0456(02)00097-2
Jamshed N, Ozair FF, Aggarwal P, Ekka M (2014) Alzheimer disease in post-menopausal women: intervene in the critical window period. J Midlife Health 5(1):38–40. https://doi.org/10.4103/0976-7800.127791
Venezia AC, Quinlan E, Roth SM (2017) A single bout of exercise increases hippocampal Bdnf: influence of chronic exercise and noradrenaline. Genes Brain Behav 16(8):800–811. https://doi.org/10.1111/gbb.12394
Dao AT, Zagaar MA, Levine AT, Salim S, Eriksen JL, Alkadhi KA (2013) Treadmill exercise prevents learning and memory impairment in Alzheimer’s disease-like pathology. Curr Alzheimer Res 10(5):507–515. https://doi.org/10.2174/1567205011310050006
Vaynman S, Ying Z, Gomez-Pinilla F (2007) The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience 144(3):825–833. https://doi.org/10.1016/j.neuroscience.2006.10.005
Zoladz JA, Pilc A (2010) The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol 61(5):533–541
Choi DH, Lee KH, Lee J (2016) Effect of exercise-induced neurogenesis on cognitive function deficit in a rat model of vascular dementia. Mol Med Rep 13(4):2981–2990. https://doi.org/10.3892/mmr.2016.4891
Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F (2004) Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 125(1):129–139. https://doi.org/10.1016/j.neuroscience.2004.01.030
Lima Giacobbo B, Doorduin J, Klein HC, Dierckx R, Bromberg E, de Vries EFJ (2019) Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol 56(5):3295–3312. https://doi.org/10.1007/s12035-018-1283-6
Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME (1997) CREB: a major mediator of neuronal neurotrophin responses. Neuron 19(5):1031–1047. https://doi.org/10.1016/s0896-6273(00)80395-5
Abel T, Kandel E (1998) Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Brain Res Rev 26(2–3):360–378. https://doi.org/10.1016/s0165-0173(97)00050-7
Park JH, Hong JH, Lee SW, Ji HD, Jung JA, Yoon KW, Lee JI, Won KS et al (2019) The effect of chronic cerebral hypoperfusion on the pathology of Alzheimer's disease: a positron emission tomography study in rats. Sci Rep 9(1):14102. https://doi.org/10.1038/s41598-019-50681-4
Bragin V, Chemodanova M, Dzhafarova N, Bragin I, Czerniawski JL, Aliev G (2005) Integrated treatment approach improves cognitive function in demented and clinically depressed patients. Am J Alzheimers Dis Other Dement 20(1):21–26. https://doi.org/10.1177/153331750502000103
Bragin V, Chemodanova M, Bragin I, Dzhafarova N, Mescher I, Chernyavskyy P, Obrenovich ME, Palacios HH et al (2012) A 60-month follow-up of a naturalistic study of integrative treatment for real-life geriatric patients with depression, dementia and multiple chronic illnesses. Open Journal of Psychiatry 2(2):129–140. https://doi.org/10.4236/ojpsych.2012.22018
Jia RX, Liang JH, Xu Y, Wang YQ (2019) Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr 19(1):181. https://doi.org/10.1186/s12877-019-1175-2
Aliev G, Bragin V (2010) Progression of cognitive function after integrated treatment approach in demented and clinically depressed patients. In: Aliev G. ea (ed) The role of oxidative stress, mitochondria failure, and cellular hypoperfusion in the context of Alzheimer disease: past, present & future. 1 edn. Publisher Research Signpost, New York, pp 317-325
Xie X, Lu W, Chen Y, Tsang CK, Liang J, Li W, Jing Z, Liao Y et al (2019) Prostaglandin E1 alleviates cognitive dysfunction in chronic cerebral hypoperfusion rats by improving hemodynamics. Front Neurosci 13:549. https://doi.org/10.3389/fnins.2019.00549
Balaban PM, Guliaeva NV (2006) Commonality of molecular mechanisms of neuroplasticity and neuropathology: Integrative approach. Ross Fiziol Zh Im I M Sechenova 92(2):145–151
Funding
Part of this study was supported by the IPAC RAS State Targets Project # 0090-2019-0005.. The study was also supported by the Russian Academic Excellence project 5-100 for the Sechenov University, Moscow, Russia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights
Animal study: Ethics approval was provided by the Local Ethical Committee, Ivanovo State Medical Academy, in accordance with the International and National Guidelines on conducting studies involving the use of experimental animals. Cervical dislocation was used to euthanize the animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chrishtop, V.V., Tomilova, I.K., Rumyantseva, T.A. et al. The Effect of Short-Term Physical Activity on the Oxidative Stress in Rats with Different Stress Resistance Profiles in Cerebral Hypoperfusion. Mol Neurobiol 57, 3014–3026 (2020). https://doi.org/10.1007/s12035-020-01930-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01930-5