Abstract
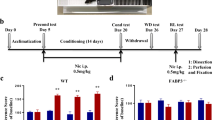
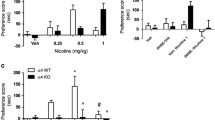
Nicotine in tobacco causes psychological dependence through its rewarding effect in the central nervous system (CNS). Although nicotine dependence is explained by dopamine receptor (DR) signaling together with nicotinic acetylcholine receptors (nAChRs), the synaptic molecular mechanism underlying the interaction between dopamine receptor and nAChRs remains unclear. Since reward signaling is mediated by dopamine receptors, we hypothesized that the dopamine D2 receptor (D2R), in part, mediates the synaptic modulation of nicotine-induced conditioned place preference (CPP) in addition to dopamine D1 receptor. To investigate the involvement of D2R, wild-type (WT) and dopamine D2 receptor knockout (D2RKO) mice were assessed using the CPP task after induction of nicotine-induced CPP. As expected, D2RKO mice failed to induce CPP behaviors after repeated nicotine administration (0.5 mg/kg). When kinase signaling was assessed in the nucleus accumbens and hippocampal CA1 region after repeated nicotine administration, both Ca2+/calmodulin-dependent protein kinase (CaMKII) and extracellular signal-regulated kinase (ERK) were upregulated in WT mice but not in D2RKO mice. Likewise, nicotine-induced CPP was associated with elevation of pro- brain-derived neurotropic factor (BDNF) and BDNF protein levels in WT mice, but not in D2RKO mice. Taken together, in addition to dopamine D1 receptor signaling, dopamine D2 receptor signaling is critical for induction of nicotine-induced CPP in mice.









Similar content being viewed by others
Abbreviations
- AcS:
-
Nucleus accumbens shell
- ACC:
-
Anterior cingulate cortex
- α4β2nAChR:
-
Alpha 4 beta 2 nicotinic acetylcholine receptors
- α7nAChRs:
-
Alpha 7 nicotinic acetylcholine receptors
- BDNF:
-
Brain-derived neurotropic factor
- CaMKII:
-
Calcium/calmodulin-dependent protein kinase II
- CHRNA4:
-
Nicotinic acetylcholine receptor (Nachr) Α4 subunit
- CPP:
-
Conditioned placed preference
- CREB:
-
cAMP response element binding
- D2RKO:
-
Dopamine D2 receptor knockout
- DLS:
-
Dorsolateral striatum
- D1R:
-
Dopamine D1 receptors
- D2R:
-
Dopamine D2 receptors
- ERK:
-
Extracellular signal-regulated kinase
- LTP:
-
Long-term potentiation
- MSN:
-
Medium spiny neurons
- NAc:
-
Nucleus accumbens
- NTRK2:
-
Neurotrophic tyrosine kinase receptor 2
- pCREB:
-
Phosphorylation cAMP response element binding
- PK:
-
Phosphokinase C
- PVDF:
-
Polyvinylidene difluoride
- PtC:
-
Parietal association cortex
- VTA:
-
Ventral tegmental area
- WT:
-
Wild type
References
Benowitz NL (1999) Nicotine addiction. Prim Care 26:611–631
Changeux J-P (2010) Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci 11:389–401. https://doi.org/10.1038/nrn2849
Leslie FM, Mojica CY, Reynaga DD (2013) Nicotinic receptors in addiction pathways. Mol Pharmacol 83:753–758. https://doi.org/10.1124/mol.112.083659
De Biasi M, Dani JA (2011) Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci 34:105–130. https://doi.org/10.1146/annurev-neuro-061010-113734
Nguyen HN, Rasmussen B, Perry DC (2003) Subtype-selective up-regulation by chronic nicotine of high- affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther 307:1090–1097. https://doi.org/10.1124/jpet.103.056408.able
Nomikos GG, Schilström B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH (2000) Role of alpha7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behav Brain Res 113:97–103. https://doi.org/10.1016/S0166-4328(00)00204-7
Missale C, Nash SR, Robinson SW et al (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225. https://doi.org/10.1152/physrev.1998.78.1.189
Gingrich JA, Caron MG (1993) Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci 16:299–321. https://doi.org/10.1146/annurev.ne.16.030193.001503
Inoue Y, Yao L, Hopf FW et al (2007) Nicotine and ethanol activate protein kinase A synergistically via G. Pharmacology 322:23–29. https://doi.org/10.1124/jpet.107.120675.jority
Baik J-H (2013) Dopamine signaling in reward-related behaviors. Front Neural Circuits 7:1–16. https://doi.org/10.3389/fncir.2013.00152
Gore BB, Zweifel LS (2013) Genetic reconstruction of dopamine D1 receptor signaling in the nucleus accumbens facilitates natural and drug reward responses. J Neurosci 33:8640–8649. https://doi.org/10.1523/JNEUROSCI.5532-12.2013
Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME (2002) D1 dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem 81:984–992. https://doi.org/10.1046/j.1471-4159.2002.00877.x
Mangiavacchi S, Wolf ME (2004) D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem 88:1261–1271. https://doi.org/10.1046/j.1471-4159.2003.02248.x
Kutlu MG, Burke D, Slade S, Hall BJ, Rose JE, Levin ED (2013) Role of insular cortex D1 and D2 dopamine receptors in nicotine self-administration in rats. Behav Brain Res 256:273–278. https://doi.org/10.1016/j.bbr.2013.08.005
Hall BJ, Slade S, Allenby C, Kutlu MG, Levin ED (2015) Neuro-anatomic mapping of dopamine D1 receptor involvement in nicotine self-administration in rats. Neuropharmacology 99:689–695. https://doi.org/10.1016/j.neuropharm.2015.03.005
Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A (2004) Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J Neurochem 90:1094–1103. https://doi.org/10.1111/j.1471-4159.2004.02574.x
McCarthy MJ, Duchemin AM, Neff NH, Hadjiconstantinou M (2012) CREB involvement in the regulation of striatal prodynorphin by nicotine. Psychopharmacology 221:143–153. https://doi.org/10.1007/s00213-011-2559-y
Novak G, Seeman P, Le FB (2010) Exposure to nicotine produces an increase in dopamine D2 high receptors: a possible mechanism for dopamine hypersensitivity. Int J Neurosci 120:691–697. https://doi.org/10.3109/00207454.2010.513462
Zhang SF, Xie CL, Wang Q, Liu ZG (2014) Interactions of CaMKII with dopamine D2 receptors: roles in levodopa-induced dyskinesia in 6-hydroxydopamine lesioned Parkinson’s rats. Sci Rep 4:1–6. https://doi.org/10.1038/srep06811
Elgersma Y (2004) Mouse genetic approaches to investigating calcium/calmodulin-dependent protein kinase II function in plasticity and cognition. J Neurosci 24:8410–8415. https://doi.org/10.1523/JNEUROSCI.3622-04.2004
Irvine EE, von Hertzen LSJ, Plattner F, Giese KP (2006) αCaMKII autophosphorylation: a fast track to memory. Trends Neurosci 29:459–465. https://doi.org/10.1016/j.tins.2006.06.009
Zlomuzica A, Machulska A, Roberts S, von Glischinski M, Rinck M, Lester KJ, Eley TC, Margraf J (2018) The dopamine D2 receptor mediates approach-avoidance tendencies in smokers. Eur Arch Psychiatry Clin Neurosci 268:261–268. https://doi.org/10.1007/s00406-017-0793-y
Takeuchi Y, Fukunaga K, Miyamoto E (2002) Activation of nuclear Ca(2+)/calmodulin-dependent protein kinase II and brain-derived neurotrophic factor gene expression by stimulation of dopamine D2 receptor in transfected NG108-15 cells. J Neurochem 82:316–328
Kamata A, Takeuchi Y, Fukunaga K (2006) Identification of the isoforms of Ca2+/calmodulin-dependent protein kinase II and expression of brain-derived neurotrophic factor mRNAs in the substantia nigra. J Neurochem 96:195–203. https://doi.org/10.1111/j.1471-4159.2005.03531.x
Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P (2001) BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411:86–89. https://doi.org/10.1038/35075076
Mössner R, Daniel S, Albert D, Heils A, Okladnova O, Schmitt A, Lesch KP (2000) Serotonin transporter function is modulated by brain-derived neurotrophic factor (BDNF) but not nerve growth factor (NGF). Neurochem Int 36:197–202. https://doi.org/10.1016/S0197-0186(99)00122-9
Vaidya V, Marek GJ, Aghajanian GK, Duman RS (1997) 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 17:2785–2795
Berninger B, Marty S, Zafra F et al (1995) GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development 121:2327–2335. https://doi.org/10.1242/dev.00351
Knipper M, da Penha Berzaghi M, Blöchl A et al (1994) Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci 6:668–671. https://doi.org/10.1111/j.1460-9568.1994.tb00312.x
Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R et al (1997) Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19:103–113. https://doi.org/10.1016/S0896-6273(00)80351-7
Carboni E, Vacca C (2002) Conditioned place preference. Drugs Abus Neurol Rev Protoc 79:481–498
Yabuki Y, Fukunaga K (2013) Oral administration of glutathione improves memory deficits following transient brain ischemia by reducing brain oxidative stress. Neuroscience 250:394–407. https://doi.org/10.1016/j.neuroscience.2013.07.017
Yabuki Y, Takahata I, Matsuo K, Owada Y, Fukunaga K (2018) Ramelteon improves post-traumatic stress disorder-like behaviors exhibited by fatty acid-binding protein 3 null mice. Mol Neurobiol 55:3577–3591. https://doi.org/10.1007/s12035-017-0587-2
Risinger FO, Oakes RA (1995) Nicotine-induced conditioned place preference and conditioned place aversion in mice. Pharmacol Biochem Behav 51:457–461. https://doi.org/10.1016/0091-3057(95)00007-J
Laviolette SR, Lauzon NM, Bishop SF, Sun N, Tan H (2008) Dopamine signaling through D1-like versus D2-like receptors in the nucleus accumbens core versus shell differentially modulates nicotine reward sensitivity. J Neurosci 28:8025–8033. https://doi.org/10.1523/JNEUROSCI.1371-08.2008
Pontieri FE, Tanda G, Orzi F, Di Chiara G (1996) Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 382:255–257
Crespo JA (2006) Activation of muscarinic and nicotinic acetylcholine receptors in the nucleus accumbens core is necessary for the acquisition of drug reinforcement. J Neurosci 26:6004–6010. https://doi.org/10.1523/JNEUROSCI.4494-05.2006
Pyakurel P, Shin M, Venton BJ (2018) Nicotinic acetylcholine receptor (nAChR) mediated dopamine release in larval Drosophila melanogaster. Neurochem Int 114:33–41. https://doi.org/10.1016/j.neuint.2017.12.012
Benowitz NL (2010) Nicotine addiction. N Engl J Med 362:2295–2303. https://doi.org/10.1056/NEJMra0809890
Brunzell DH, McIntosh JM (2012) Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology 37:1134–1143. https://doi.org/10.1038/npp.2011.299
Govind AP, Vezina P, Green WN (2009) Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol 78:756–765. https://doi.org/10.1016/j.bcp.2009.06.011
Besson M, Granon S, Mameli-Engvall M, Cloez-Tayarani I, Maubourguet N, Cormier A, Cazala P, David V et al (2007) Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proc Natl Acad Sci 104:8155–8160. https://doi.org/10.1073/pnas.0702698104
Heishman SJ, Kleykamp BA, Singleton EG (2010) Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology 210:453–469. https://doi.org/10.1007/s00213-010-1848-1
Mclaughlin I, Dani JA, De Biasi M (2015) The neuropharmacology of nicotine dependence. 24:99–123. https://doi.org/10.1007/978-3-319-13482-6
Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW (2000) Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol 393:51–58. https://doi.org/10.1016/S0014-2999(00)00005-4
McGranahan TM, Patzlaff NE, Grady SR et al (2011) 4 2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci 31:10891–10902. https://doi.org/10.1523/JNEUROSCI.0937-11.2011
Wooltorton JRA, Pidoplichko VI, Broide RS, Dani JA (2003) Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci 23:3176–3185
Garzón M, Duffy AM, Chan J, Lynch MK, Mackie K, Pickel VM (2013) Dopamine D2 and acetylcholine α7 nicotinic receptors have subcellular distributions favoring mediation of convergent signaling in the mouse ventral tegmental area. Neuroscience 252:126–143. https://doi.org/10.1016/j.neuroscience.2013.08.008
Dani JA (2003) Roles of dopamine signaling in nicotine addiction. Mol Psychiatry 8:255–256. https://doi.org/10.1038/sj.mp.4001284
Ehlinger DG, Burke JC, McDonald CG et al (2017) Nicotine-induced and D1-receptor-dependent dendritic remodeling in a subset of dorsolateral striatum medium spiny neurons. Neuroscience 356:242–254. https://doi.org/10.1016/j.neuroscience.2017.05.036
Guy EG, Fletcher PJ (2014) Responding for a conditioned reinforcer, and its enhancement by nicotine, is blocked by dopamine receptor antagonists and a 5-HT2Creceptor agonist but not by a 5-HT2Areceptor antagonist. Pharmacol Biochem Behav 125:40–47. https://doi.org/10.1016/j.pbb.2014.08.006
Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Matsumoto G, Ono T (2003) Dopamine D2 receptor-knockout changed accumbens neural response to prediction of reward associated with place in mice. Int Congr Ser 1250:493–508. https://doi.org/10.1016/S0531-5131(03)00966-X
Kelly MA, Rubinstein M, Phillips TJ et al (1998) Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci 18:3470–3479. https://doi.org/10.1523/jneurosci.4936-09.2010
Kutlu MG, Gould TJ (2016) Nicotinic modulation of hippocampal cell signaling and associated effects on learning and memory. Physiol Behav 155:162–171. https://doi.org/10.1016/j.physbeh.2015.12.008
Picconi B (2004) Abnormal Ca2+-calmodulin-dependent protein kinase II function mediates synaptic and motor deficits in experimental parkinsonism. J Neurosci 24:5283–5291. https://doi.org/10.1523/JNEUROSCI.1224-04.2004
Fukunaga K, Shioda N (2012) Novel dopamine D2 receptor signaling through proteins interacting with the third cytoplasmic loop. Mol Neurobiol 45:144–152. https://doi.org/10.1007/s12035-011-8227-8
Tahara S, Fukuda K, Kodama H, Kato T, Miyoshi S, Ogawa S (2001) Potassium channel blocker activates extracellular signal-regulated kinases through Pyk2 and epidermal growth factor receptor in rat cardiomyocytes. J Am Coll Cardiol 38:1554–1563
Luttrell LM, Daaka Y, Lefkowitz RJ (1999) Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol 11:177–183
Chiamulera C, Di Chio M, Tedesco V et al (2008) Nicotine-induced phosphorylation of phosphorylated cyclic AMP response element-binding protein (pCREB) in hippocampal neurons is potentiated by agrin. Neurosci Lett 442:234–238. https://doi.org/10.1016/j.neulet.2008.07.025
Wu G-Y, Deisseroth K, Tsien RW (2001) Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci 98:2808–2813. https://doi.org/10.1073/pnas.051634198
Brunzell DH, Mineur YS, Neve RL, Picciotto MR (2009) Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology 34:1993–2001. https://doi.org/10.1038/npp.2009.11
Hall FS, Drgonova J, Goeb M, Uhl GR (2003) Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology 28:1485–1490. https://doi.org/10.1038/sj.npp.1300192
Hensler JG, Ladenheim EE, Lyons WE (2003) Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/−) mice. J Neurochem 85:1139–1147. https://doi.org/10.1046/j.1471-4159.2003.01748.x
Kenny PJ, File SE, Rattray M (2000) Acute nicotine decreases, and chronic nicotine increases the expression of brain-derived neurotrophic factor mRNA in rat hippocampus. Brain Res Mol Brain Res 85:234–238
Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O’Dowd BF, George SR (2009) Calcium signaling cascade links dopamine D1–D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci 106:21377–21382. https://doi.org/10.1073/pnas.0903676106
Peterson DJ, Gill WD, Dose JM, Hoover DB, Pauly JR, Cummins ED, Burgess KC, Brown RW (2017) The effects of nicotine in the neonatal quinpirole rodent model of psychosis: neural plasticity mechanisms and nicotinic receptor changes. Behav Brain Res 325:17–24. https://doi.org/10.1016/j.bbr.2017.02.029
Beuten J, Ma JZ, Payne TJ, Dupont RT, Lou XY, Crews KM, Elston RC, Li MD (2007) Association of specific haplotypes of neurotrophic tyrosine kinase receptor 2 gene (NTRK2) with vulnerability to nicotine dependence in African-Americans and European-Americans. Biol Psychiatry 61:48–55. https://doi.org/10.1016/j.biopsych.2006.02.023
Ohira K, Hayashi M (2009) A new aspect of the TrkB signaling pathway in neural plasticity. Curr Neuropharmacol 7:276–285. https://doi.org/10.2174/157015909790031210
Kumar V (2005) Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci 25:11288–11299. https://doi.org/10.1523/JNEUROSCI.2284-05.2005
Lee BG, Anastasia A, Hempstead BL, Lee FS, Blendy JA (2015) Effects of the BDNF Val66Met polymorphism on anxiety-like behavior following nicotine withdrawal in mice. Nicotine Tob Res 17:1428–1435. https://doi.org/10.1093/ntr/ntv047
Egan MF, Kojima M, Callicott JH et al (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function and its val/met polymorphism in human memory and hippocampal function and suggest val/met exerts these effects by impacting intracellular. Cell 112:257–269. https://doi.org/10.1016/j.solener.2017.10.050
Jamal M, Van der Does W, Elzinga BM et al (2015) Association between smoking, nicotine dependence, and BDNF Val66Met polymorphism with BDNF concentrations in serum. Nicotine Tob Res 17:323–329. https://doi.org/10.1093/ntr/ntu151
Faure P, Tolu S, Valverde S, Naudé J (2014) Role of nicotinic acetylcholine receptors in regulating dopamine neuron activity. Neuroscience 282:86–100. https://doi.org/10.1016/j.neuroscience.2014.05.040
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This research was funded by the Grant Indonesia Endowment Fund for Education (LPDP) to G.W. and Indonesia Endowment fund for Education (LPDP) and Smoking Research Foundation and the Project of Translational and Clinical Research Core Centers, AMED, Japan (JP17dm0107071 and JP18dm0107071 to K.F.).
Author information
Authors and Affiliations
Contributions
K.F. and G.W. conceived and coordinated the study and wrote the paper. G.W. performed and analyzed the experiment shown in figures. Y.S. provided technical assistance. K.F. and G.W. reviewed the results. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
All experimental animal procedures were approved by the Committee on Animal Experiments at Tohoku University, and studies were conducted in accordance with committee guidelines.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wilar, G., Shinoda, Y., Sasaoka, T. et al. Crucial Role of Dopamine D2 Receptor Signaling in Nicotine-Induced Conditioned Place Preference. Mol Neurobiol 56, 7911–7928 (2019). https://doi.org/10.1007/s12035-019-1635-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1635-x