Abstract
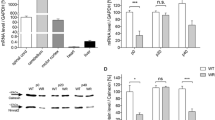
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder with no cure, and elucidation of the mechanisms mediating neuronal death in this neuropathology is crucial to develop effective treatments. It has recently been demonstrated in animal models that the Wnt family of proteins is involved in this neuropathology, although its potential involvement in case of humans is almost unknown. We analyzed the expression of Wnt signaling components in healthy and ALS human spinal cords by quantitative RT-PCR, and we found that most Wnt ligands, modulators, receptors, and co-receptors were expressed in healthy controls. Moreover, we observed clear alterations in the mRNA expression of different components of this family of proteins in human spinal cord tissue from ALS cases. Specifically, we detected a significant increase in the mRNA levels of Wnt3, Wnt4, Fz2, and Fz8, together with several non-significant increases in the mRNA expression of other genes such as Wnt2b, Wnt5a, Fz3, Lrp5, and sFRP3. Based on these observations and on previous reports of studies performed in animal models, we evaluated with immunohistochemistry the protein expression patterns of Fz2 and Fz5 receptors and their main ligand Wnt5a in control samples and ALS cases. No substantial changes were observed in Fz5 protein expression pattern in ALS samples. However, we detected an increase in the amount of Fz2+ astrocytes in the borderline between gray and white matter at the ventral horn in ALS samples. Finally, Wnt5a expression was observed in neurons and astrocytes in both control and ALS samples, although Wnt5a immunolabeling in astroglial cells was significantly increased in ALS spinal cords in the same region where changes in Fz2 were observed. Altogether, these observations strongly suggest that the Wnt family of proteins, and more specifically Fz2 and Wnt5a, might be involved in human ALS pathology.





Similar content being viewed by others
References
Cleveland DW, Rothstein JD (2001) From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2(11):806–819. https://doi.org/10.1038/35097565
Morgan S, Orrell RW (2016) Pathogenesis of amyotrophic lateral sclerosis. Br Med Bull 119:87–98. https://doi.org/10.1093/bmb/ldw026
Moloney EB, de Winter F, Verhaagen J (2014) ALS as a distal axonopathy: molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front Neurosci 8:252. https://doi.org/10.3389/fnins.2014.00252
Rothstein JD (2009) Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol 65(Suppl 1):S3–S9. https://doi.org/10.1002/ana.21543
Garbuzova-Davis S, Rodrigues MC, Hernandez-Ontiveros DG, Louis MK, Willing AE, Borlongan CV, Sanberg PR (2011) Amyotrophic lateral sclerosis: a neurovascular disease. Brain Res 1398:113–125. https://doi.org/10.1016/j.brainres.2011.04.049
Tan RH, Ke YD, Ittner LM, Halliday GM (2017) ALS/FTLD: experimental models and reality. Acta Neuropathol 133:177–196. https://doi.org/10.1007/s00401-016-1666-6
Gros-Louis F, Gaspar C, Rouleau GA (2006) Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim Biophys Acta 1762(11–12):956–972. https://doi.org/10.1016/j.bbadis.2006.01.004
Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V (1996) Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 347(9013):1425–1431
Miller RG, Mitchell JD, Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 3:CD001447. https://doi.org/10.1002/14651858.CD001447.pub3
Al-Chalabi A, Calvo A, Chio A, Colville S, Ellis CM, Hardiman O, Heverin M, Howard RS et al (2014) Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol 13(11):1108–1113. https://doi.org/10.1016/S1474-4422(14)70219-4
Do-Ha D, Buskila Y, Ooi L (2017) Impairments in motor neurons, interneurons and astrocytes contribute to hyperexcitability in ALS: underlying mechanisms and paths to therapy. Mol Neurobiol 55:1410–1418. https://doi.org/10.1007/s12035-017-0392-y
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotrophic lateral sclerosis. Lancet 377(9769):942–955. https://doi.org/10.1016/S0140-6736(10)61156-7
Shaw PJ (2005) Molecular and cellular pathways of neurodegeneration in motor neurone disease. J Neurol Neurosurg Psychiatry 76(8):1046–1057. https://doi.org/10.1136/jnnp.2004.048652
Pasinelli P, Brown RH (2006) Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat Rev Neurosci 7(9):710–723. https://doi.org/10.1038/nrn1971
Gladman M, Cudkowicz M, Zinman L (2012) Enhancing clinical trials in neurodegenerative disorders: lessons from amyotrophic lateral sclerosis. Curr Opin Neurol 25(6):735–742. https://doi.org/10.1097/WCO.0b013e32835a309d
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127(3):469–480. https://doi.org/10.1016/j.cell.2006.10.018
Megason SG, McMahon AP (2002) A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129(9):2087–2098
Ciani L, Salinas PC (2005) WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci 6(5):351–362. https://doi.org/10.1038/nrn1665
Inestrosa NC, Arenas E (2010) Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 11(2):77–86. https://doi.org/10.1038/nrn2755
Gonzalez-Fernandez C, Arevalo-Martin A, Paniagua-Torija B, Ferrer I, Rodriguez FJ, Garcia-Ovejero D (2016) Wnts are expressed in the ependymal region of the adult spinal cord. Mol Neurobiol 54:6342–6355. https://doi.org/10.1007/s12035-016-0132-8
Inestrosa NC, Toledo EM (2008) The role of Wnt signaling in neuronal dysfunction in Alzheimer’s disease. Mol Neurodegener 3:9. https://doi.org/10.1186/1750-1326-3-9
Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ (2011) Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One 6(11):e27000. https://doi.org/10.1371/journal.pone.0027000
Gonzalez P, Fernandez-Martos CM, Gonzalez-Fernandez C, Arenas E, Rodriguez FJ (2012) Spatio-temporal expression pattern of frizzled receptors after contusive spinal cord injury in adult rats. PLoS One 7(12):e50793. https://doi.org/10.1371/journal.pone.0050793
Gonzalez P, Fernandez-Martos CM, Arenas E, Rodriguez FJ (2013) The Ryk receptor is expressed in glial and fibronectin-expressing cells after spinal cord injury. J Neurotrauma 30(10):806–817. https://doi.org/10.1089/neu.2012.2613
Gonzalez-Fernandez C, Fernandez-Martos CM, Shields S, Arenas E, Rodriguez FJ (2013) Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma 31(6):565–581. https://doi.org/10.1089/neu.2013.3067
Lambert C, Cisternas P, Inestrosa NC (2015) Role of Wnt signaling in central nervous system injury. Mol Neurobiol 53:2297–2311. https://doi.org/10.1007/s12035-015-9138-x
Gonzalez P, Rodriguez FJ (2017) Analysis of the expression of the Wnt family of proteins and its modulatory role on cytokine expression in non activated and activated astroglial cells. Neurosci Res 114:16–29. https://doi.org/10.1016/j.neures.2016.08.003
Tapia-Rojas C, Inestrosa NC (2017) Wnt signaling loss accelerates the appearance of neuropathological hallmarks of Alzheimer’s disease in J20-APP transgenic and wild-type mice. J Neurochem 144:443–465. https://doi.org/10.1111/jnc.14278
Pinto C, Cardenas P, Osses N, Henriquez JP (2013) Characterization of Wnt/beta-catenin and BMP/Smad signaling pathways in an in vitro model of amyotrophic lateral sclerosis. Front Cell Neurosci 7:239. https://doi.org/10.3389/fncel.2013.00239
Li X, Guan Y, Chen Y, Zhang C, Shi C, Zhou F, Yu L, Juan J et al (2013) Expression of Wnt5a and its receptor Fzd2 is changed in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. Int J Clin Exp Pathol 6(7):1245–1260
Yu L, Guan Y, Wu X, Chen Y, Liu Z, Du H, Wang X (2013) Wnt signaling is altered by spinal cord neuronal dysfunction in amyotrophic lateral sclerosis transgenic mice. Neurochem Res 38(9):1904–1913. https://doi.org/10.1007/s11064-013-1096-y
Tury A, Tolentino K, Zou Y (2014) Altered expression of atypical PKC and Ryk in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Dev Neurobiol 74(8):839–850. https://doi.org/10.1002/dneu.22137
Wang S, Guan Y, Chen Y, Li X, Zhang C, Yu L, Zhou F, Wang X (2013) Role of Wnt1 and Fzd1 in the spinal cord pathogenesis of amyotrophic lateral sclerosis-transgenic mice. Biotechnol Lett 35(8):1199–1207. https://doi.org/10.1007/s10529-013-1199-1
Chen Y, Guan Y, Zhang Z, Liu H, Wang S, Yu L, Wu X, Wang X (2012) Wnt signaling pathway is involved in the pathogenesis of amyotrophic lateral sclerosis in adult transgenic mice. Neurol Res 34(4):390–399. https://doi.org/10.1179/1743132812Y.0000000027
Chen Y, Guan Y, Liu H, Wu X, Yu L, Wang S, Zhao C, Du H et al (2012) Activation of the Wnt/beta-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem Biophys Res Commun 420(2):397–403. https://doi.org/10.1016/j.bbrc.2012.03.006
McLoon LK, Harandi VM, Brannstrom T, Andersen PM, Liu JX (2014) Wnt and extraocular muscle sparing in amyotrophic lateral sclerosis. Invest Ophthalmol Vis Sci 55(9):5482–5496. https://doi.org/10.1167/iovs.14-14886
Gonzalez-Fernandez C, Mancuso R, Del Valle J, Navarro X, Rodriguez FJ (2016) Wnt signaling alteration in the spinal cord of amyotrophic lateral sclerosis transgenic mice: special focus on Frizzled-5 cellular expression pattern. PLoS One 11(5):e0155867. https://doi.org/10.1371/journal.pone.0155867
Hendrickx M, Leyns L (2008) Non-conventional Frizzled ligands and Wnt receptors. Develop Growth Differ 50(4):229–243. https://doi.org/10.1111/j.1440-169X.2008.01016.x
Fradkin LG, Dura JM, Noordermeer JN (2010) Ryks: new partners for Wnts in the developing and regenerating nervous system. Trends Neurosci 33(2):84–92. https://doi.org/10.1016/j.tins.2009.11.005
Minami Y, Oishi I, Endo M, Nishita M (2010) Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev Dyn 239(1):1–15. https://doi.org/10.1002/dvdy.21991
Schulte G (2010) International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol Rev 62(4):632–667. https://doi.org/10.1124/pr.110.002931
Niehrs C (2012) The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol 13(12):767–779. https://doi.org/10.1038/nrm3470
Cadigan KM, Liu YI (2006) Wnt signaling: complexity at the surface. J Cell Sci 119(Pt 3):395–402. https://doi.org/10.1242/jcs.02826
Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10(7):468–477. https://doi.org/10.1038/nrm2717
Widelitz R (2005) Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors 23(2):111–116. https://doi.org/10.1080/08977190500125746
Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116(Pt 13):2627–2634. https://doi.org/10.1242/jcs.00623
Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J (2008) Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 121(Pt 6):737–746. https://doi.org/10.1242/jcs.026096
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112(4):389–404. https://doi.org/10.1007/s00401-006-0127-z
Mancuso R, Navarro X (2017) Sigma-1 receptor in motoneuron disease. Adv Exp Med Biol 964:235–254. https://doi.org/10.1007/978-3-319-50174-1_16
Garcia-Ovejero D, Arevalo-Martin A, Paniagua-Torija B, Florensa-Vila J, Ferrer I, Grassner L, Molina-Holgado E (2015) The ependymal region of the adult human spinal cord differs from other species and shows ependymoma-like features. Brain 138(Pt 6):1583–1597. https://doi.org/10.1093/brain/awv089
Johann S, Heitzer M, Kanagaratnam M, Goswami A, Rizo T, Weis J, Troost D, Beyer C (2015) NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 63(12):2260–2273. https://doi.org/10.1002/glia.22891
Paniagua-Torija B, Arevalo-Martin A, Molina-Holgado E, Molina-Holgado F, Garcia-Ovejero D (2015) Spinal cord injury induces a long-lasting upregulation of interleukin-1beta in astrocytes around the central canal. Neuroscience 284:283–289. https://doi.org/10.1016/j.neuroscience.2014.10.013
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676–682. https://doi.org/10.1038/nmeth.2019
Paniagua-Torija B, Arevalo-Martin A, Ferrer I, Molina-Holgado E, Garcia-Ovejero D (2015) CB1 cannabinoid receptor enrichment in the ependymal region of the adult human spinal cord. Sci Rep 5:17745. https://doi.org/10.1038/srep17745
Barbeito AG, Mesci P, Boillee S (2010) Motor neuron-immune interactions: the vicious circle of ALS. J Neural Transm 117(8):981–1000. https://doi.org/10.1007/s00702-010-0429-0
Lee J, Hyeon SJ, Im H, Ryu H, Kim Y, Ryu H (2016) Astrocytes and microglia as non-cell autonomous players in the pathogenesis of ALS. Exp Neurobiol 25(5):233–240. https://doi.org/10.5607/en.2016.25.5.233
Kawamata T, Akiyama H, Yamada T, McGeer PL (1992) Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am J Pathol 140(3):691–707
Schiffer D, Cordera S, Cavalla P, Migheli A (1996) Reactive astrogliosis of the spinal cord in amyotrophic lateral sclerosis. J Neurol Sci 139(Suppl):27–33
Vucic S, Kiernan MC (2009) Pathophysiology of neurodegeneration in familial amyotrophic lateral sclerosis. Curr Mol Med 9(3):255–272
Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G et al (2004) Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer's brain. J Neurosci 24(26):6021–6027. https://doi.org/10.1523/JNEUROSCI.1381-04.2004
Wei H, Qin ZH, Senatorov VV, Wei W, Wang Y, Qian Y, Chuang DM (2001) Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of Huntington’s disease. Neuroscience 106(3):603–612
Godin JD, Poizat G, Hickey MA, Maschat F, Humbert S (2010) Mutant huntingtin-impaired degradation of beta-catenin causes neurotoxicity in Huntington’s disease. EMBO J 29(14):2433–2445. https://doi.org/10.1038/emboj.2010.117
L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Cossetti C, D'Adamo P, Zardini E et al (2011) Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiol Dis 41(2):508–527. https://doi.org/10.1016/j.nbd.2010.10.023
Parish CL, Castelo-Branco G, Rawal N, Tonnesen J, Sorensen AT, Salto C, Kokaia M, Lindvall O et al (2008) Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J Clin Invest 118(1):149–160. https://doi.org/10.1172/JCI32273
Yuan S, Shi Y, Tang SJ (2012) Wnt signaling in the pathogenesis of multiple sclerosis-associated chronic pain. J NeuroImmune Pharmacol 7(4):904–913. https://doi.org/10.1007/s11481-012-9370-3
Xie C, Li Z, Zhang GX, Guan Y (2014) Wnt signaling in remyelination in multiple sclerosis: friend or foe? Mol Neurobiol 49(3):1117–1125. https://doi.org/10.1007/s12035-013-8584-6
McCord M, Mukouyama YS, Gilbert MR, Jackson S (2017) Targeting WNT signaling for multifaceted glioblastoma therapy. Front Cell Neurosci 11:318. https://doi.org/10.3389/fncel.2017.00318
Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y (2008) Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci 28(33):8376–8382. https://doi.org/10.1523/JNEUROSCI.1939-08.2008
Miyashita T, Koda M, Kitajo K, Yamazaki M, Takahashi K, Kikuchi A, Yamashita T (2009) Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma 26(7):955–964. https://doi.org/10.1089/neu.2008.0776
Chen Y, Wang Q, Wang Q, Liu H, Zhou F, Zhang Y, Yuan M, Zhao C et al (2017) DDX3 binding with CK1epsilon was closely related to motor neuron degeneration of ALS by affecting neurite outgrowth. Am J Transl Res 9(10):4627–4639
de Oliveira GP, Maximino JR, Maschietto M, Zanoteli E, Puga RD, Lima L, Carraro DM, Chadi G (2014) Early gene expression changes in skeletal muscle from SOD1(G93A) amyotrophic lateral sclerosis animal model. Cell Mol Neurobiol 34(3):451–462. https://doi.org/10.1007/s10571-014-0029-x
Bhinge A, Namboori SC, Zhang X, VanDongen AMJ, Stanton LW (2017) Genetic correction of SOD1 mutant iPSCs reveals ERK and JNK activated AP1 as a driver of neurodegeneration in amyotrophic lateral sclerosis. Stem Cell Rep 8(4):856–869. https://doi.org/10.1016/j.stemcr.2017.02.019
Niu LJ, Xu RX, Zhang P, Du MX, Jiang XD (2012) Suppression of Frizzled-2-mediated Wnt/Ca(2)(+) signaling significantly attenuates intracellular calcium accumulation in vitro and in a rat model of traumatic brain injury. Neuroscience 213:19–28. https://doi.org/10.1016/j.neuroscience.2012.03.057
Halleskog C, Dijksterhuis JP, Kilander MB, Becerril-Ortega J, Villaescusa JC, Lindgren E, Arenas E, Schulte G (2012) Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J Neuroinflammation 9:111. https://doi.org/10.1186/1742-2094-9-111
Yamanaka K, Komine O (2017) The multi-dimensional roles of astrocytes in ALS. Neurosci Res 126:31–38. https://doi.org/10.1016/j.neures.2017.09.011
Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94(4):1077–1098. https://doi.org/10.1152/physrev.00041.2013
Rossi D (2015) Astrocyte physiopathology: at the crossroads of intercellular networking, inflammation and cell death. Prog Neurobiol 130:86–120. https://doi.org/10.1016/j.pneurobio.2015.04.003
Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S (2007) Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10(5):615–622. https://doi.org/10.1038/nn1876
Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estevez AG, Beckman JS (2004) A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev 47(1–3):263–274. https://doi.org/10.1016/j.brainresrev.2004.05.003
Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A (2010) Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J 29(1):41–54. https://doi.org/10.1038/emboj.2009.322
Bazhin AV, Tambor V, Dikov B, Philippov PP, Schadendorf D, Eichmuller SB (2010) cGMP-phosphodiesterase 6, transducin and Wnt5a/Frizzled-2-signaling control cGMP and Ca(2+) homeostasis in melanoma cells. Cell Mol Life Sci 67(5):817–828. https://doi.org/10.1007/s00018-009-0214-0
Ding S, Xu Z, Yang J, Liu L, Huang X, Wang X, Zhuge Q (2017) The involvement of the decrease of astrocytic Wnt5a in the cognitive decline in minimal hepatic encephalopathy. Mol Neurobiol 54(10):7949–7963. https://doi.org/10.1007/s12035-016-0216-5
Varela-Nallar L, Alfaro IE, Serrano FG, Parodi J, Inestrosa NC (2010) Wingless-type family member 5A (Wnt-5a) stimulates synaptic differentiation and function of glutamatergic synapses. Proc Natl Acad Sci U S A 107(49):21164–21169. https://doi.org/10.1073/pnas.1010011107
Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC (2009) Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem 284(23):15857–15866. https://doi.org/10.1074/jbc.M808986200
Li B, Zhong L, Yang X, Andersson T, Huang M, Tang SJ (2011) WNT5A signaling contributes to Abeta-induced neuroinflammation and neurotoxicity. PLoS One 6(8):e22920. https://doi.org/10.1371/journal.pone.0022920
Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, Leigh PN, Banati RB (2004) Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis 15(3):601–609. https://doi.org/10.1016/j.nbd.2003.12.012
Alexianu ME, Kozovska M, Appel SH (2001) Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology 57(7):1282–1289
Hall ED, Oostveen JA, Gurney ME (1998) Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia 23(3):249–256
Keller AF, Gravel M, Kriz J (2009) Live imaging of amyotrophic lateral sclerosis pathogenesis: disease onset is characterized by marked induction of GFAP in Schwann cells. Glia 57(10):1130–1142. https://doi.org/10.1002/glia.20836
Levine JB, Kong J, Nadler M, Xu Z (1999) Astrocytes interact intimately with degenerating motor neurons in mouse amyotrophic lateral sclerosis (ALS). Glia 28(3):215–224
Halleskog C, Schulte G (2013) WNT-3A and WNT-5A counteract lipopolysaccharide-induced pro-inflammatory changes in mouse primary microglia. J Neurochem 125(6):803–808. https://doi.org/10.1111/jnc.12250
Zhu A, Shen L, Xu L, Chen W, Huang Y (2017) Suppression of Wnt5a, but not Wnts, relieves chronic post-thoracotomy pain via anti-inflammatory modulation in rats. Biochem Biophys Res Commun 493(1):474–480. https://doi.org/10.1016/j.bbrc.2017.08.167
Valencia J, Martinez VG, Hidalgo L, Hernandez-Lopez C, Canseco NM, Vicente A, Varas A, Sacedon R (2014) Wnt5a signaling increases IL-12 secretion by human dendritic cells and enhances IFN-gamma production by CD4+ T cells. Immunol Lett 162(1 Pt A):188–199. https://doi.org/10.1016/j.imlet.2014.08.015
Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G (2008) Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol 28(3):504–510. https://doi.org/10.1161/ATVBAHA.107.157438
Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E et al (2006) The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 108(3):965–973. https://doi.org/10.1182/blood-2005-12-5046
Kumawat K, Gosens R (2016) WNT-5A: signaling and functions in health and disease. Cell Mol Life Sci 73(3):567–587. https://doi.org/10.1007/s00018-015-2076-y
Halleskog C, Mulder J, Dahlstrom J, Mackie K, Hortobagyi T, Tanila H, Kumar Puli L, Farber K et al (2011) WNT signaling in activated microglia is proinflammatory. Glia 59(1):119–131. https://doi.org/10.1002/glia.21081
Libro R, Bramanti P, Mazzon E (2016) The role of the Wnt canonical signaling in neurodegenerative diseases. Life Sci 158:78–88. https://doi.org/10.1016/j.lfs.2016.06.024
Biechele TL, Camp ND, Fass DM, Kulikauskas RM, Robin NC, White BD, Taraska CM, Moore EC et al (2010) Chemical-genetic screen identifies riluzole as an enhancer of Wnt/beta-catenin signaling in melanoma. Chem Biol 17(11):1177–1182. https://doi.org/10.1016/j.chembiol.2010.08.012
Acknowledgments
We would like to thank Sandra Vázquez and Virginia Pérez for their outstanding technical help, as well as Dr. Daniel García-Ovejero from the Group of Neuroinflammation for sharing with us his deep knowledge and methodology on the human histology used in the study. We are extremely grateful to all individuals who agreed to donate their tissues to research.
Funding
This work was funded by the Fondo de Investigaciones Sanitarias (FIS) (Grant PI12-02895, co-funded by Fondo Europeo de Desarrollo Regional (FEDER)) from the Instituto de Salud Carlos III (ISCIII).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement on the Welfare of Animals
This article does not contain any studies with animals performed by any of the authors.
Conflict of Interest
The authors declare they have no conflict of interests.
Statement on Sample Extraction and Processing from ALS Patients
Postmortem samples from all individual participants were obtained with written informed consent prior to inclusion in the study, which has been conducted according to 1964 Declaration of Helsinki principles and its later amendments, following the ethical rule of the Hospital Universitari de Bellvitge (Spain) and according to the Directive 2004/23/EC of the European Parliament and of the Council. All samples were handled after approval by the Clinical Research Ethical Committee (CEIC) in Toledo (Spain) and in accordance with Spanish law and International Guidelines (LOPD 15/1999; RD 1720/2007; 1964 Helsinki declaration and its later amendments or comparable ethical standards).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Pre-incubation of Fz2, Fz5 and Wnt5a antibodies with their corresponding blocking peptides. The pre-incubation was performed with 10-fold weight/weight excess for Fz2 and Fz5, and 20-fold weight/weight excess for Wnt5a. Representative images showing the immunostaining blockade of Fz2 (b1- b3), Fz5 (a1- a3) and Wnt5a (c1- c3). Scale bars = 100μm. (PNG 1436 kb)
Rights and permissions
About this article
Cite this article
González-Fernández, C., Gonzalez, P., Andres-Benito, P. et al. Wnt Signaling Alterations in the Human Spinal Cord of Amyotrophic Lateral Sclerosis Cases: Spotlight on Fz2 and Wnt5a. Mol Neurobiol 56, 6777–6791 (2019). https://doi.org/10.1007/s12035-019-1547-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1547-9