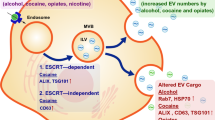
Abstract
Extracellular vesicles (EVs) are small vesicles secreted by cells and are known to carry sub-cellular components including microRNA, proteins, and lipids. Due to their ability to transport cargo between cells, EVs have been identified as important regulators of various pathophysiological conditions and can therefore influence treatment outcomes. In particular, the significance of microRNAs in EV-mediated cell-cell communication is well-documented. While the influence of EVs and the cargo delivered by EVs has been extensively reviewed in other neurological disorders, the available literature on the potential role of EVs in the pathophysiology of drug addiction has not been reviewed. Hence, in this article, the known effects of commonly abused drugs (ethanol, nicotine, opiates, cocaine, and cannabinoids) on EV secretion have been reviewed. In addition, the potential role of drugs of abuse in affecting the delivery of EV-packaged microRNAs, and the subsequent impact on neuronal health and continued drug dependence, has been discussed.

Similar content being viewed by others
References
Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 33(3):967–978. https://doi.org/10.1016/0092-8674(83)90040-5
Harding CV, Heuser JE, Stahl PD (2013) Exosomes: Looking back three decades and into the future. J Cell Biol 200(4):367–371. https://doi.org/10.1083/jcb.201212113
Wen C, Seeger RC, Fabbri M, Wang L, Wayne AS, Jong AY (2017) Biological roles and potential applications of immune cell-derived extracellular vesicles. J Extracell Vesicles 6(1):1400370. https://doi.org/10.1080/20013078.2017.1400370
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: Composition, biogenesis and function. Nat Rev Immunol 2(8):569–579. https://doi.org/10.1038/nri855
Simons M, Raposo G (2009) Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol 21(4):575–581. https://doi.org/10.1016/j.ceb.2009.03.007
Von Bartheld CS, Altick AL (2011) Multivesicular bodies in neurons: Distribution, protein content, and trafficking functions. Prog Neurobiol 93(3):313–340. https://doi.org/10.1016/j.pneurobio.2011.01.003
Zhou L, Qi XL, Xu MX, Mao Y, Liu ML, Song HM (2014) Microparticles: New light shed on the understanding of venous thromboembolism. Acta Pharmacol Sin 35(9):1103–1110. https://doi.org/10.1038/aps.2014.73
Ahn YS (2005) Cell-derived microparticles: 'Miniature envoys with many faces'. J Thromb Haemost 3(5):884–887. https://doi.org/10.1111/j.1538-7836.2005.01347.x
Huotari J, Helenius A (2011) Endosome maturation. EMBO J 30(17):3481–3500. https://doi.org/10.1038/emboj.2011.286
Bartel DP (2004) MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116(2):281–297. https://doi.org/10.1016/S0092-8674(04)00045-5
Starega-Roslan J, Krol J, Koscianska E, Kozlowski P, Szlachcic WJ, Sobczak K, Krzyzosiak WJ (2011) Structural basis of microRNA length variety. Nucleic Acids Res 39(1):257–268. https://doi.org/10.1093/nar/gkq727
Cocucci E, Meldolesi J (2015) Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol 25(6):364–372. https://doi.org/10.1016/j.tcb.2015.01.004
Hessvik NP, Llorente A (2017) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75(2):193–208. https://doi.org/10.1007/s00018-017-2595-9
Mulcahy LA, Pink RC, Carter DR (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3. https://doi.org/10.3402/jev.v3.24641
Soung YH, Ford S, Zhang V, Chung J (2017) Exosomes in Cancer Diagnostics. Cancers (Basel) 9 (1). https://doi.org/10.3390/cancers9010008
Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM (2010) Exosomes: Fit to deliver small RNA. Commun Integr Biol 3(5):447–450. https://doi.org/10.4161/cib.3.5.12339
Barile L, Vassalli G (2017) Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther 174:63–78. https://doi.org/10.1016/j.pharmthera.2017.02.020
Jonas S, Izaurralde E (2015) Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16(7):421–433. https://doi.org/10.1038/nrg3965
Bartel DP (2009) MicroRNAs: Target recognition and regulatory functions. Cell 136(2):215–233. https://doi.org/10.1016/j.cell.2009.01.002
Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S (2015) Exosome and exosomal microRNA: Trafficking, sorting, and function. Genomics Proteomics Bioinformatics 13(1):17–24. https://doi.org/10.1016/j.gpb.2015.02.001
Jovicic A, Gitler AD (2017) Distinct repertoires of microRNAs present in mouse astrocytes compared to astrocyte-secreted exosomes. PLoS One 12(2):e0171418. https://doi.org/10.1371/journal.pone.0171418
Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG et al (2017) MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 48(3):747–753. https://doi.org/10.1161/STROKEAHA.116.015204
Aryani A, Denecke B (2016) Exosomes as a Nanodelivery system: A key to the future of Neuromedicine? Mol Neurobiol 53(2):818–834. https://doi.org/10.1007/s12035-014-9054-5
Levy E (2017) Exosomes in the diseased brain: First insights from in vivo studies. Front Neurosci 11:142. https://doi.org/10.3389/fnins.2017.00142
Sarko DK, McKinney CE (2017) Exosomes: Origins and therapeutic potential for neurodegenerative disease. Front Neurosci 11:82. https://doi.org/10.3389/fnins.2017.00082
Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, Starmann J, Macas J et al (2014) Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol 12(6):e1001874. https://doi.org/10.1371/journal.pbio.1001874
Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K, Brkic M, Demeestere D et al (2016) Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med 8(10):1162–1183. https://doi.org/10.15252/emmm.201606271
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M (2013) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33(11):1711–1715. https://doi.org/10.1038/jcbfm.2013.152
Couch Y, Akbar N, Roodselaar J, Evans MC, Gardiner C, Sargent I, Romero IA, Bristow A et al (2017) Circulating endothelial cell-derived extracellular vesicles mediate the acute phase response and sickness behaviour associated with CNS inflammation. Sci Rep 7(1):9574. https://doi.org/10.1038/s41598-017-09710-3
Hazleton I, Yates A, Dale A, Roodselaar J, Akbar N, Ruitenberg M, Anthony DC, Couch Y (2017) Exacerbation of acute traumatic brain injury by circulating extracellular vesicles. J Neurotrauma. https://doi.org/10.1089/neu.2017.5049
Guo L, Guo N (2015) Exosomes: Potent regulators of tumor malignancy and potential bio-tools in clinical application. Crit Rev Oncol Hematol 95(3):346–358. https://doi.org/10.1016/j.critrevonc.2015.04.002
Takahashi RU, Prieto-Vila M, Hironaka A, Ochiya T (2017) The role of extracellular vesicle microRNAs in cancer biology. Clin Chem Lab Med 55(5):648–656. https://doi.org/10.1515/cclm-2016-0708
Ellwanger JH, Veit TD, Chies JAB (2017) Exosomes in HIV infection: A review and critical look. Infection Gen Evolution : J Mol Epidemiol Evol Gen Infect Dis 53:146–154. https://doi.org/10.1016/j.meegid.2017.05.021
Raab-Traub N, Dittmer DP (2017) Viral effects on the content and function of extracellular vesicles. Nat Rev Microbiol 15(9):559–572. https://doi.org/10.1038/nrmicro.2017.60
Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD (2011) Up-regulation of microRNAs in brain of human alcoholics. Alcohol Clin Exp Res 35(11):1928–1937. https://doi.org/10.1111/j.1530-0277.2011.01544.x
Most D, Workman E, Harris RA (2014) Synaptic adaptations by alcohol and drugs of abuse: Changes in microRNA expression and mRNA regulation. Front Mol Neurosci 7:85. https://doi.org/10.3389/fnmol.2014.00085
Nunez YO, Mayfield RD (2012) Understanding alcoholism through microRNA signatures in brains of human alcoholics. Front Genet 3:43. https://doi.org/10.3389/fgene.2012.00043
Saha B, Momen-Heravi F, Kodys K, Szabo G (2016) MicroRNA cargo of extracellular vesicles from alcohol-exposed monocytes signals naive monocytes to differentiate into M2 macrophages. J Biol Chem 291(1):149–159. https://doi.org/10.1074/jbc.M115.694133
Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC et al (2016) Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol 64(3):651–660. https://doi.org/10.1016/j.jhep.2015.11.020
Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN (2008) Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron 59(2):274–287. https://doi.org/10.1016/j.neuron.2008.05.032
Mandal C, Halder D, Jung KH, Chai YG (2017) Maternal alcohol consumption and altered miRNAs in the developing fetus: Context and future perspectives. J Appl Toxicol : JAT 38(1):100–107. https://doi.org/10.1002/jat.3504
Salomon C, Guanzon D, Scholz-Romero K, Longo S, Correa P, Illanes SE, Rice GE (2017) Placental exosomes as early biomarker of preeclampsia - potential role of exosomal microRNAs across gestation. J Clin Endocrinol Metab 102(9):3182–3194. https://doi.org/10.1210/jc.2017-00672
Salomon C, Rice GE (2017) Role of exosomes in placental homeostasis and pregnancy disorders. Prog Mol Biol Transl Sci 145:163–179. https://doi.org/10.1016/bs.pmbts.2016.12.006
Benedikter BJ, Volgers C, van Eijck PH, Wouters EFM, Savelkoul PHM, Reynaert NL, Haenen G, Rohde GGU et al (2017) Cigarette smoke extract induced exosome release is mediated by depletion of exofacial thiols and can be inhibited by thiol-antioxidants. Free Radic Biol Med 108:334–344. https://doi.org/10.1016/j.freeradbiomed.2017.03.026
Li CJ, Liu Y, Chen Y, Yu D, Williams KJ, Liu ML (2013) Novel proteolytic microvesicles released from human macrophages after exposure to tobacco smoke. Am J Pathol 182(5):1552–1562. https://doi.org/10.1016/j.ajpath.2013.01.035
Fujita Y, Araya J, Ito S, Kobayashi K, Kosaka N, Yoshioka Y, Kadota T, Hara H et al (2015) Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J Extracell Vesicles 4(1):28388. https://doi.org/10.3402/jev.v4.28388
Fujita Y, Araya J, Ochiya T (2015) Extracellular vesicles in smoking-related lung diseases. Oncotarget 6(41):43144–43145. https://doi.org/10.18632/oncotarget.6556
Mulle C, Vidal C, Benoit P, Changeux JP (1991) Existence of different subtypes of nicotinic acetylcholine receptors in the rat habenulo-interpeduncular system. J Neurosci 11(8):2588–2597
Lee S, Woo J, Kim YS, Im HI (2015) Integrated miRNA-mRNA analysis in the habenula nuclei of mice intravenously self-administering nicotine. Sci Rep 5(1):12909. https://doi.org/10.1038/srep12909
Hogan EM, Casserly AP, Scofield MD, Mou Z, Zhao-Shea R, Johnson CW, Tapper AR, Gardner PD (2014) miRNAome analysis of the mammalian neuronal nicotinic acetylcholine receptor gene family. RNA 20(12):1890–1899. https://doi.org/10.1261/rna.034066.112
Benwell ME, Balfour DJ, Anderson JM (1988) Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem 50(4):1243–1247. https://doi.org/10.1111/j.1471-4159.1988.tb10600.x
Barbierato M, Zusso M, Skaper SD, Giusti P (2015) MicroRNAs: Emerging role in the endogenous mu opioid system. CNS & Neurol Disorder Drug Targets 14(2):239–250. https://doi.org/10.2174/1871527314666150116123932
Hwang CK, Wagley Y, Law PY, Wei LN, Loh HH (2012) MicroRNAs in opioid pharmacology. J NeuroImmune Pharmacol 7(4):808–819. https://doi.org/10.1007/s11481-011-9323-2
He Y, Yang C, Kirkmire CM, Wang ZJ (2010) Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci 30(30):10251–10258. https://doi.org/10.1523/JNEUROSCI.2419-10.2010
Haug BH, Hald OH, Utnes P, Roth SA, Lokke C, Flaegstad T, Einvik C (2015) Exosome-like extracellular vesicles from MYCN-amplified neuroblastoma cells contain oncogenic miRNAs. Anticancer Res 35(5):2521–2530
Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, Jiang X, Hou D et al (2015) Targeted exosome-mediated delivery of opioid receptor mu siRNA for the treatment of morphine relapse. Sci Rep 5(1):17543. https://doi.org/10.1038/srep17543
Hu G, Yao H, Chaudhuri AD, Duan M, Yelamanchili SV, Wen H, Cheney PD, Fox HS et al (2012) Exosome-mediated shuttling of microRNA-29 regulates HIV tat and morphine-mediated neuronal dysfunction. Cell Death Dis 3(8):e381. https://doi.org/10.1038/cddis.2012.114
Smith ACW, Kenny PJ (2017) MicroRNAs regulate synaptic plasticity underlying drug addiction. Genes Brain Behav. https://doi.org/10.1111/gbb.12424
Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C et al (2010) Striatal microRNA controls cocaine intake through CREB signalling. Nature 466(7303):197–202. https://doi.org/10.1038/nature09202
Quinn RK, Brown AL, Goldie BJ, Levi EM, Dickson PW, Smith DW, Cairns MJ, Dayas CV (2015) Distinct miRNA expression in dorsal striatal subregions is associated with risk for addiction in rats. Transl Psychiatry 5(2):e503. https://doi.org/10.1038/tp.2014.144
Onaivi ES, Schanz N, Lin ZC (2014) Psychiatric disturbances regulate the innate immune system in CSF of conscious mice. Transl Psychiatry 4(3):e367. https://doi.org/10.1038/tp.2014.5
Carone C, Genedani S, Leo G, Filaferro M, Fuxe K, Agnati LF (2015) In vitro effects of cocaine on tunneling nanotube formation and extracellular vesicle release in glioblastoma cell cultures. J Mol Neurosci : MN 55(1):42–50. https://doi.org/10.1007/s12031-014-0365-9
Zlebnik NE, Cheer JF (2016) Drug-induced alterations of endocannabinoid-mediated plasticity in brain reward regions. J Neurosci 36(40):10230–10238. https://doi.org/10.1523/JNEUROSCI.1712-16.2016
Hayase T (2017) Epigenetic mechanisms associated with addiction-related behavioural effects of nicotine and/or cocaine: Implication of the endocannabinoid system. Behav Pharmacol 28(7):493–511. https://doi.org/10.1097/FBP.0000000000000326
Chiarlone A, Borner C, Martin-Gomez L, Jimenez-Gonzalez A, Garcia-Concejo A, Garcia-Bermejo ML, Lorente M, Blazquez C et al (2016) MicroRNA let-7d is a target of cannabinoid CB1 receptor and controls cannabinoid signaling. Neuropharmacology 108:345–352. https://doi.org/10.1016/j.neuropharm.2016.05.007
Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, Matteoli M, Maccarrone M et al (2015) Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep 16(2):213–220. https://doi.org/10.15252/embr.201439668
Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R (2014) Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles 3(1):24722. https://doi.org/10.3402/jev.v3.24722
Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R (2016) Astrocytes as secretory cells of the central nervous system: Idiosyncrasies of vesicular secretion. EMBO J 35(3):239–257. https://doi.org/10.15252/embj.201592705
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345. https://doi.org/10.1038/nbt.1807
Ebrahimkhani S, Vafaee F, Young PE, Hur SSJ, Hawke S, Devenney E, Beadnall H, Barnett MH et al (2017) Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Rep 7(1):14293. https://doi.org/10.1038/s41598-017-14301-3
Acknowledgements
This review work was not supported by any extramural financial source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rao, P.S.S., O’Connell, K. & Finnerty, T.K. Potential Role of Extracellular Vesicles in the Pathophysiology of Drug Addiction. Mol Neurobiol 55, 6906–6913 (2018). https://doi.org/10.1007/s12035-018-0912-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0912-4