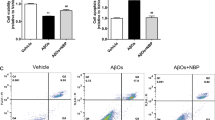
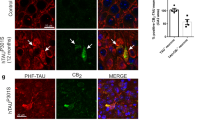
Abstract
Although several studies have shown that type-2 cannabinoid receptor (CB2R) is involved in Alzheimer’s disease (AD) pathology, the effects of CB2R on AD-like tau abnormal phosphorylation and its underlying mechanism remain unclear. Herein, we employed the CB2R−/− mice as the animal model to explore roles of CB2R in regulating tau phosphorylation and brain function. We found that CB2R−/− mice display AD-like tau hyperphosphorylation, hippocampus-dependent memory impairment, increase of GSK3β activity, decrease of AMPK and Sirt1 activity and mitochondria dysfunction. Interestingly, AICAR or resveratrol (AMPK agonist) could efficiently rescue most alternations caused by solo deletion of CB2R in CB2R−/− mice. Moreover, JWH133, a selective agonist of CB2R, reduces phosphorylation of tau and GSK3β activity in HEK293 tau cells, but the effects of JWH133 on phosphorylation of tau and GSK3β disappeared while blocking AMPK activity with compound C or Prkaa2-RNAi. Taken together, our study indicated that deletion of CB2R induces behavior damage and AD-like pathological alternation via AMPK/GSK3β pathway. These findings proved that CB2R/AMPK/GSK3β pathway can be a promising new drug target for AD.







Similar content being viewed by others
References
Pedrós I, Petrov D, Allgaier M, Sureda F, Barroso E, Beas-Zarate C, Auladell C, Pallàs M et al (2014) Early alterations in energy metabolism in the hippocampus of APPswe/PS1dE9 mouse model of Alzheimer’s disease. Biochim Biophys Acta (BBA) - Mol Basis Dis 1842(9):1556–1566. doi:10.1016/j.bbadis.2014.05.025
Yin F, Sancheti H, Patil I, Cadenas E (2016) Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol Med 100:108–122. doi:10.1016/j.freeradbiomed.2016.04.200
Salminen A, Haapasalo A, Kauppinen A, Kaarniranta K, Soininen H, Hiltunen M (2015) Impaired mitochondrial energy metabolism in Alzheimer’s disease: Impact on pathogenesis via disturbed epigenetic regulation of chromatin landscape. Prog Neurobiol 131:1–20. doi:10.1016/j.pneurobio.2015.05.001
Selkoe DJ (1994) Cell biology of the amyloid beta-protein precursor and the mechanism of Alzheimer’s disease. Annu Rev Cell Biol 10(1):373–403. doi:10.1146/annurev.cb.10.110194.002105
Arendt T, Stieler JT, Holzer M (2016) Tau and tauopathies. Brain Res Bull 126(Pt 3):238–292. doi:10.1016/j.brainresbull.2016.08.018
Kim GH, Kim JE, Rhie SJ, Yoon S (2015) The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol 24(4):325–340. doi:10.5607/en.2015.24.4.325
Cabezas-Opazo FA, Vergara-Pulgar K, Perez MJ, Jara C, Osorio-Fuentealba C, Quintanilla RA (2015) Mitochondrial dysfunction contributes to the pathogenesis of Alzheimer’s disease. Oxidative Med Cell Longev 2015:509654. doi:10.1155/2015/509654
Guerriero F, Sgarlata C, Francis M, Maurizi N, Faragli A, Perna S, Rondanelli M, Rollone M et al (2016) Neuroinflammation, immune system and Alzheimer disease: searching for the missing link. Aging Clin Exp Res. doi:10.1007/s40520-016-0637-z
Latek D, Kolinski M, Ghoshdastider U, Debinski A, Bombolewski R, Plazinska A, Jozwiak K, Filipek S (2011) Modeling of ligand binding to G protein coupled receptors: cannabinoid CB1, CB2 and adrenergic β2AR. J Mol Model 17(9):2353–2366. doi:10.1007/s00894-011-0986-7
Mallipeddi S, Janero DR, Zvonok N, Makriyannis A Functional selectivity at G-protein coupled receptors: Advancing cannabinoid receptors as drug targets. Biochem Pharmacol. doi:10.1016/j.bcp.2016.11.014
Onaivi ES, Chaudhuri G, Abaci AS, Parker M, Manier DH, Martin PR, Hubbard JR (1999) Expression of cannabinoid receptors and their gene transcripts in human blood cells. Prog Neuro-Psychopharmacol Biol Psychiatry 23(6):1063–1077. doi:10.1016/S0278-5846(99)00052-4
Cabral GA, Griffin-Thomas L (2009) Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med 11. doi:10.1017/S1462399409000957
Lanciego JL, Barroso-Chinea P, Rico AJ, Conte-Perales L, Callen L, Roda E, Gomez-Bautista V, Lopez IP et al (2011) Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol 25(1):97–104. doi:10.1177/0269881110367732
Li Y, Kim J (2015) Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience 311:253–267. doi:10.1016/j.neuroscience.2015.10.041
Skaper SD, Buriani A, Dal Toso R, Petrelli L, Romanello S, Facci L, Leon A (1996) The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. Proc Natl Acad Sci U S A 93(9):3984–3989. doi:10.1073/pnas.93.9.3984
Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, Gardner EL, Wu J et al (2014) Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A 111(46):E5007–E5015. doi:10.1073/pnas.1413210111
Zhang HY, Bi GH, Li X, Li J, Qu H, Zhang SJ, Li CY, Onaivi ES et al (2015) Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology 40(4):1037–1051. doi:10.1038/npp.2014.297
Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, Sagredo O, Benito C et al (2009) Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain 132(Pt 11):3152–3164. doi:10.1093/brain/awp239
Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH et al (2011) Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci 14(9):1160–1166. doi:10.1038/nn.2874
Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J (2012) Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. Br J Pharmacol 165(4):951–964. doi:10.1111/j.1476-5381.2011.01625.x
Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, Navarrete F, Manzanares J (2011) Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology 36(7):1489–1504. doi:10.1038/npp.2011.34
Aso E, Ferrer I (2016) CB2 cannabinoid receptor as potential target against Alzheimer’s disease. Front Neurosci 10:243. doi:10.3389/fnins.2016.00243
Aso E, Juves S, Maldonado R, Ferrer I (2013) CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AbetaPP/PS1 mice. J Alzheimer’s Dis: JAD 35(4):847–858. doi:10.3233/JAD-130137
Koppel J, Vingtdeux V, Marambaud P, d’Abramo C, Jimenez H, Stauber M, Friedman R, Davies P (2014) CB2 receptor deficiency increases amyloid pathology and alters tau processing in a transgenic mouse model of Alzheimer’s disease. Mol Med 20:29–36. doi:10.2119/molmed.2013.00140.revised
Wu J, Bie B, Yang H, Xu JJ, Brown DL, Naguib M (2013) Activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol Aging 34(3):791–804. doi:10.1016/j.neurobiolaging.2012.06.011
Aso E, Andres-Benito P, Carmona M, Maldonado R, Ferrer I (2016) Cannabinoid receptor 2 participates in amyloid-beta processing in a mouse model of Alzheimer’s disease but plays a minor role in the therapeutic properties of a cannabis-based medicine. J Alzheimer’s Dis: JAD 51(2):489–500. doi:10.3233/JAD-150913
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13(4):251–262. doi:10.1038/nrm3311
Domise M, Vingtdeux V (2016) AMPK in neurodegenerative diseases. EXS 107:153–177. doi:10.1007/978-3-319-43589-3_7
Frasch MG (2014) Putative role of AMPK in fetal adaptive brain shut-down: linking metabolism and inflammation in the brain. Front Neurol 5:150. doi:10.3389/fneur.2014.00150
Choi IY, Ju C, Anthony Jalin AM, Lee DI, Prather PL, Kim WK (2013) Activation of cannabinoid CB2 receptor-mediated AMPK/CREB pathway reduces cerebral ischemic injury. Am J Pathol 182(3):928–939. doi:10.1016/j.ajpath.2012.11.024
Clark JK, Furgerson M, Crystal JD, Fechheimer M, Furukawa R, Wagner JJ (2015) Alterations in synaptic plasticity coincide with deficits in spatial working memory in presymptomatic 3xTg-AD mice. Neurobiol Learn Mem 125:152–162. doi:10.1016/j.nlm.2015.09.003
Mei Y, Jiang C, Wan Y, Lv J, Jia J, Wang X, Yang X, Tong Z (2015) Aging-associated formaldehyde-induced norepinephrine deficiency contributes to age-related memory decline. Aging Cell 14(4):659–668. doi:10.1111/acel.12345
Li Y, Kim J (2016) CB2 cannabinoid receptor knockout in mice impairs contextual long-term memory and enhances spatial working memory. Neural Plast 2016:9817089. doi:10.1155/2016/9817089
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1):47–60. doi:10.1016/0165-0270(84)90007-4
Thornton C, Bright Nicola J, Sastre M, Muckett Phillip J, Carling D (2011) AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid β-peptide exposure. Biochem J 434(3):503
Shah SA, Yoon GH, Chung SS, Abid MN, Kim TH, Lee HY, Kim MO (2016) Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer’s disease neuropathological deficits. Mol Psychiatry. doi:10.1038/mp.2016.23
Vingtdeux V, Davies P, Dickson DW, Marambaud P (2011) AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathol 121(3):337–349. doi:10.1007/s00401-010-0759-x
Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML (2005) Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci 25(8):1904–1913. doi:10.1523/JNEUROSCI.4540-04.2005
Solas M, Francis PT, Franco R, Ramirez MJ (2013) CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol Aging 34(3):805–808. doi:10.1016/j.neurobiolaging.2012.06.005
Ahmad R, Postnov A, Bormans G, Versijpt J, Vandenbulcke M, Van Laere K (2016) Decreased in vivo availability of the cannabinoid type 2 receptor in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 43(12):2219–2227. doi:10.1007/s00259-016-3457-7
Garcia-Gutierrez MS, Ortega-Alvaro A, Busquets-Garcia A, Perez-Ortiz JM, Caltana L, Ricatti MJ, Brusco A, Maldonado R et al (2013) Synaptic plasticity alterations associated with memory impairment induced by deletion of CB2 cannabinoid receptors. Neuropharmacology 73:388–396. doi:10.1016/j.neuropharm.2013.05.034
Schmole AC, Lundt R, Ternes S, Albayram O, Ulas T, Schultze JL, Bano D, Nicotera P et al (2015) Cannabinoid receptor 2 deficiency results in reduced neuroinflammation in an Alzheimer’s disease mouse model. Neurobiol Aging 36(2):710–719. doi:10.1016/j.neurobiolaging.2014.09.019
Cheng Y, Dong Z, Liu S (2014) β-Caryophyllene Ameliorates the Alzheimer-Like Phenotype in APP/PS1 Mice through CB2 Receptor Activation and the PPARγ Pathway. Pharmacology 94(1–2):1–12
Davies SP, Hawley SA, Woods A, Carling D, Haystead TAJ, Hardie DG (1994) Purification of the AMP-activated protein kinase on ATP-γ-Sepharose and analysis of its subunit structure. Eur J Biochem 223(2):351–357. doi:10.1111/j.1432-1033.1994.tb19001.x
Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG (1996) Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271(44):27879–27887. doi:10.1074/jbc.271.44.27879
Park Y-J, Ko JW, Jang Y, Kwon YH (2013) Activation of AMP-activated protein kinase alleviates homocysteine-mediated neurotoxicity in SH-SY5Y cells. Neurochem Res 38(8):1561–1571. doi:10.1007/s11064-013-1057-5
Greco SJ, Hamzelou A, Johnston JM, Smith MA, Ashford JW, Tezapsidis N (2011) Leptin boosts cellular metabolism by activating AMPK and the sirtuins to reduce tau phosphorylation and β-amyloid in neurons. Biochem Biophys Res Commun 414(1):170–174. doi:10.1016/j.bbrc.2011.09.050
Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, Liu LB, Wu K et al (2015) AMPK activation ameliorates Alzheimer’s disease-like pathology and spatial memory impairment in a streptozotocin-induced Alzheimer’s disease model in rats. J Alzheimer’s Dis: JAD 43(3):775–784. doi:10.3233/JAD-140564
Liu F, Liang Z, Shi J, Yin D, El-Akkad E, Grundke-Iqbal I, Iqbal K, Gong C-X (2006) PKA modulates GSK-3β- and cdk5-catalyzed phosphorylation of tau in site- and kinase-specific manners. FEBS Lett 580(26):6269–6274. doi:10.1016/j.febslet.2006.10.033
Köhler C, Dinekov M, Götz J (2013) Active glycogen synthase kinase-3 and tau pathology-related tyrosine phosphorylation in pR5 human tau transgenic mice. Neurobiol Aging 34(5):1369–1379. doi:10.1016/j.neurobiolaging.2012.11.010
Sontag JM, Nunbhakdi-Craig V, White CL 3rd, Halpain S, Sontag E (2012) The protein phosphatase PP2A/Balpha binds to the microtubule-associated proteins tau and MAP2 at a motif also recognized by the kinase Fyn: implications for tauopathies. J Biol Chem 287(18):14984–14993. doi:10.1074/jbc.M111.338681
Plattner F, Angelo M, Giese KP (2006) The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem 281(35):25457–25465. doi:10.1074/jbc.M603469200
Horike N, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Kamata H, Nishiyama K, Uchijima Y et al (2008) AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem 283(49):33902–33910. doi:10.1074/jbc.M802537200
Kim HS, Moon S, Paik JH, Shin DW, Kim LS, Park CS, Ha J, Kang JH (2015) Activation of the 5′-AMP-activated protein kinase in the cerebral cortex of young senescence-accelerated P8 mice and association with GSK3beta- and PP2A-dependent inhibition of p-tau(3)(9)(6) expression. J Alzheimer’s Dis: JAD 46(1):249–259. doi:10.3233/JAD-150035
Han Y, Wang Q, Song P, Zhu Y, Zou M-H (2010) Redox regulation of the AMP-activated protein kinase. PLoS One 5(11):e15420. doi:10.1371/journal.pone.0015420
Karuppagounder SS, Pinto JT, Xu H, Chen H-L, Beal MF, Gibson GE (2009) Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int 54(2):111–118. doi:10.1016/j.neuint.2008.10.008
Sin TK, Yu AP, Yung BY, Yip SP, Chan LW, Wong CS, Rudd JA, Siu PM (2015) Effects of long-term resveratrol-induced SIRT1 activation on insulin and apoptotic signalling in aged skeletal muscle. Acta Diabetol 52(6):1063–1075. doi:10.1007/s00592-015-0767-3
Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L, Sun Y, Zhang Z et al (2012) Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J Nutr Biochem 23(9):1100–1112. doi:10.1016/j.jnutbio.2011.06.003
Yang Y-j HL, Y-p X, C-y J, Miao C, Yang C-q, Yuan M, Wang L (2016) Resveratrol suppresses glial activation and alleviates trigeminal neuralgia via activation of AMPK. J Neuroinflammation 13(1):84. doi:10.1186/s12974-016-0550-6
Um J-H, Park S-J, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B et al (2010) AMP-activated protein kinase–deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59(3):554
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP et al (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39(3):409–421. doi:10.1016/S0896-6273(03)00434-3
Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, Wang XC, Chen XQ et al (2007) Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci U S A 104(9):3591–3596. doi:10.1073/pnas.0609303104
Li XH, Lv BL, Xie JZ, Liu J, Zhou XW, Wang JZ (2012) AGEs induce Alzheimer-like tau pathology and memory deficit via RAGE-mediated GSK-3 activation. Neurobiol Aging 33(7):1400–1410. doi:10.1016/j.neurobiolaging.2011.02.003
ABB FV, Malhotra R, De* A (2014) IHC profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. doi:10.1371/journal.pone.0096801.t001
Peng CX, Hu J, Liu D, Hong XP, Wu YY, Zhu LQ, Wang JZ (2013) Disease-modified glycogen synthase kinase-3beta intervention by melatonin arrests the pathology and memory deficits in an Alzheimer’s animal model. Neurobiol Aging 34(6):1555–1563. doi:10.1016/j.neurobiolaging.2012.12.010
Kass MD, Rosenthal MC, Pottackal J, McGann JP (2013) Fear learning enhances neural responses to threat-predictive sensory stimuli. Science 342(6164):1389–1392. doi:10.1126/science.1244916
Li XH, Xie JZ, Jiang X, Lv BL, Cheng XS, Du LL, Zhang JY, Wang JZ et al (2012) Methylglyoxal induces tau hyperphosphorylation via promoting AGEs formation. NeuroMolecular Med 14(4):338–348. doi:10.1007/s12017-012-8191-0
Acknowledgements
This work was supported by the National Nature Scientific Fund of China (81671262 and 81171196) and Self-innovation fund of HUST and Integrated Innovative Team for Major Human Diseases Program of Tongji Medical College, HUST. The authors would like to thank Dr. Nancy E. Buckley for providing CB2R KO mice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Fig. S1
Inhibition of AMPK or CB2R causes tau hyperphosphorylation in HEK 293 tau cells. HEK293 tau cells were transfected with AMPK shRNA or scramble shRNA for 48 h, and then given AM630 for 24 h, the cell lysates were prepared for western blot. (a) The expression level of total tau (Tau5), phosphorylated tau (pT231, pS396), phosphorylated AMPK (pT172-AMPK) and CB2R. (b) Quantitation of the immunoreactivities to the antibodies shown in (a) respectively. The experiments were independently performed at least three times (n = 3), one-way ANOVA with a post hoc Tukey’s test, *p < 0.05, **p < 0.01 versus Scr; #P < 0.05, ##P < 0.01 versus Scr + AM630. Data were expressed as mean ± SEM (JPEG 332 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Liu, BJ., Cao, Y. et al. Deletion of Type-2 Cannabinoid Receptor Induces Alzheimer’s Disease-Like Tau Pathology and Memory Impairment Through AMPK/GSK3β Pathway. Mol Neurobiol 55, 4731–4744 (2018). https://doi.org/10.1007/s12035-017-0676-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0676-2