Abstract
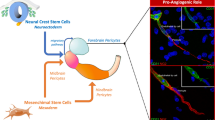
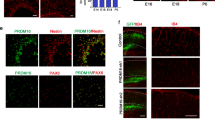
Neuroangiogenesis in the developing central nervous system is controlled by interactions between endothelial cells (ECs) and radial glia (RG) neural stem cells, although RG-derived molecules implicated in these events are not fully known. Here, we investigated the role of RG-secreted TGF-β1, in angiogenesis in the developing cerebral cortex. By isolation of murine microcapillary brain endothelial cells (MBECs), we demonstrate that conditioned medium from RG cultures (RG-CM) promoted MBEC migration and formation of vessel-like structures in vitro, in a TGF-β1-dependent manner. These events were followed by endothelial regulation of GPR124 and BAI-1 gene expression by RG-CM. Proteome profile of RG-CM identified angiogenesis-related molecules IGFBP2/3, osteopontin, endostatin, SDF1, fractalkine, TIMP1/4, Ang-1, pentraxin3, and Cyr61, some of them modulated by TGF-β1 induction. In vivo gain and loss of function assays targeting RG cells demonstrates a specific TGF-β1-dependent control of blood vessels branching in the cerebral cortex. Together, our results point to TGF-β1 signaling pathway as a potential mediator of the RG-EC interactions and shed light to the key role of RG in paving the brain vascular network.






Similar content being viewed by others
References
Walchli T, Wacker A, Frei K, Regli L, Schwab ME, Hoerstrup SP, Gerhardt H, Engelhardt B (2015) Wiring the vascular network with neural cues: a CNS perspective. Neuron 87:271–296
Abbot NJ (2005) Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 25:5–23
Kim JH, Park JA, Lee SW, Kim WJ, Yu YS, Kim KW (2006) Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol 39:339–345
Takahashi T, Takase Y, Yoshino T, Saito D, Tadokoro R, Takahashi Y (2015) Angiogenesis in the developing spinal cord: blood vessel exclusion from neural progenitor region is mediated by VEGF and its antagonists. PLoS One 10:e0116119
Rakic P (1999) Neurobiology. Discriminating migrations. Nature 400:315–316
Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR (2001) Neurons derived from radial glial cells establish radial units in neocortex. Nature 409:714–720
Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184
Ortega JA, Alcantara S (2010) BDNF/MAPK/ERK-induced BMP7 expression in the developing cerebral cortex induces premature radial glia differentiation and impairs neuronal migration. Cereb Cortex 20:2132–2144
Barbabé-Heider F, Wasylnka JA, Fernandes KJ, Porsche C, Sendtner M, Kaplan DR, Miller FD (2005) Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 48:253–265
Stipursky J, Gomes FCA (2007) TGF-beta1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development. Glia 55:1023–1033
Stipursky J, Francis D, Gomes FCA (2012) Activation of MAPK/PI3K/SMAD pathways by TGF-beta(1) controls differentiation of radial glia into astrocytes in vitro. Dev Neurosci 34:68–81
Stipursky J, Francis D, Dezonne RS, Bérgamo de Araújo AP, Souza L, Moraes CA, Gomes FCA (2014) TGF-β1 promotes cerebral cortex radial glia-astrocyte differentiation in vivo. Front Cell Neurosci 8:393
Kessaris N, Pringle N, Richardson WD (2008) Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Philos Trans R Soc Lond Ser B Biol Sci 363:71–85
Morest DK, Silver J (2003) Precursors of neurons, neuroglia, and ependymal cells in the CNS: what are they? Where are they from? How do they get where they are going? Glia 43:6–18
Ma S, Kwon HJ, Johng H, Zang GK, Huang Z (2013) Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS Biol 11:e1001469
Virgintino D, Errede M, Robertson D, Girolamo F, Masciandaro A, Bertossi M (2003) VEGF expression is developmentally regulated during human brain angiogenesis. Histochem Cell Biol 119:227–232
Hellbach N, Weise SC, Vezzali R, Wahane SD, Heidrich S, Roidl D, Pruszak J, Esser JS et al (2012) Neural deletion of Tgfbr2 impairs angiogenesis through an altered secretome. Hum Mol Genet 23:6177–6190
Massagué J (1998) TGF-beta signal transduction. Annu Rev Biochem 67:753–791
Massagué J, Gomis RR (2006) The logic of TGFbeta signaling. FEBS Lett 580:2811–2820
Gantus MA, Alves LM, Stipursky J, Souza EC, Teodoro AJ, Alves TR, Carvalho DP, Martinez AM et al (2011) Estradiol modulates TGF-β1 expression and its signaling pathway in thyroid stromal cells. Mol Cell Endocrinol 337:71–79
Brionne TC, Tesseur I, Masliah E, Wyss-Coray T (2003) Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40:1133–1145
Siegenthaler JA, Miller MW (2004) Transforming growth factor beta1 modulates cell migration in rat cortex: effects of ethanol. Cereb Cortex 14:791–802
Romão LF, de Sousa VO, Neto VM, Gomes FCAA (2008) Glutamate activates GFAP gene promoter from cultured astrocytes through TGF-beta1 pathways. J Neurochem 106:746–756
Sousa VO, Romão L, Moura Neto V, Gomes FCA (2004) Glial fibrillary acidic protein gene promoter is differently modulated by transforming growth factor-beta 1 in astrocytes from distinct brain regions. Eur J Neurosci 19:1721–1730
Diniz LP, Almeida JC, Tortelli V, Vargas-Lopes C, Setti-Perdigão P, Stipursky J, Kahn SA, Romão LF et al (2012) Astrocyte-induced synaptogenesis is mediated by transforming growth factor beta signaling through modulation of D-serine levels in cerebral cortex neurons. J Biol Chem 287:41432–41445
Diniz LP, Tortelli V, Garcia MN, Araújo AP, Melo HM, Silva GS, Felice FG, Alves-Leon SV et al (2014) Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia 62:1917–1931
Pepper MS (1997) Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev 8:21–43
Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K et al (2000) Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A 97:2626–2631
Nguyen HL, Lee YJ, Shin J, Lee E, Park SO, Mccarty JH, Oh SP (2011) TGF-beta signaling in endothelial cells, but not neuroepithelial cells, is essential for cerebral vascular development. Lab Investig 91:1554–1563
Garcia CM, Daland DC, Massingham LJ, D'amore PA (2004) Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties. Brain Res Dev Brain Res 152:25–38
Goumans MJ, Valdimarstottir G, Itoh S, Rosendahl A, Sideras P, Ten Dijke P (2002) Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 21:1743–1753
Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM et al (2004) Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J 23:4018–4028
Merwin JR, Anderson JM, Kocher O, Van Itallie CM, Madri JA (1990) Transforming growth factor beta 1 modulates extracellular matrix organization and cell-cell junctional complex formation during in vitro angiogenesis. J Cell Physiol 142:117–128
Pardali E, Goumans MJ, Ten Dijke P (2010) Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol 20:556–567
Ray BN, Lee NY, How T, Blobe GC (2010) ALK5 phosphorylation of the endoglin cytoplasmic domain regulates Smad1/5/8 signaling and endothelial cell migration. Carcinogenesis 31:435–441
Wang HH, Su CH, Wu YJ, Li JY, Tseng YM, Lin YC, Hsieh CL, Tsai CH et al (2013) Reduction of connexin43 in human endothelial progenitor cells impairs the angiogenic potential. Angiogenesis 16:553–560
Mecha M, Rabadan MA, Pena-Melian A, Valencia M, Mondejar T, Blanco MJ (2008) Expression of TGF-betas in the embryonic nervous system: analysis of interbalance between isoforms. Dev Dyn 237:1709–1717
Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, Tanaka K, Niwa M (2009) A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int 54:253–263
Stins MF, Prasadarao NV, Zhou J, Arditi M, Kim KS (1997) Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell Dev Biol Anim 4:243–247
Livak KJ, Schmittgen TDL (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 4:402–408
Walantus W, Castaneda D, Elias L, Kriegstein A (2007) In utero intraventricular injection and electroporation of E15 mouse embryos. J Vis Exp 6:239
Zudaire E, Gambardella L, Kurcz C, Vermeren S (2011) A computational tool for quantitative analysis of vascular networks. PLoS One 6:e27385
McCarty JH (2005) Cell biology of the neurovascular unit: implications for drug delivery across the blood–brain barrier. Assay Drug Dev Technol 3:89–95
Hatzopoulos AK, Folkman J, Vasile E, Eiselen GK, Rosenberg RD (1998) Isolation and characterization of endothelial progenitor cells from mouse embryos. Development 125:1457–1468
Lamszus K, Schmidt NO, Ergun S, Westphal M (1999) Isolation and culture of human neuromicrovascular endothelial cells for the study of angiogenesis in vitro. J Neurosci Res 55:370–381
Lippmann, E.S., Weidenfeller, C., ; Svendsen, C.N. and Shusta, E.V. (2011). Blood-brain barrier modeling with co-cultured neural progenitor cell-derived astrocytes and neurons. J Neurochem 119, 507–520.
Liu Y, Xue Q, Tang Q, Hoe M, Qi H, Chen G, Chen W, Zhang J et al (2013) A simple method for isolating and culturing the rat brain microvascular endothelial cells. Microvasc Res 90:199–205
Navone SE, Marfia G, Invernici G, Cristini S, Nava S, Balbi S, Sangiorgi S, Ciusani E et al (2013) Isolation and expansion of human and mouse brain microvascular endothelial cells. Nat Protoc 8:1680–1693
Wu Z, Hofman FM, Zlokovic BV (2003) A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J Neurosci Methods 130:53–63
Marelli-Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler RI (2000) Isolation of endothelial cells from murine tissue. J Immunol Methods 244:205–215
Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, Peri G, Breviario F et al (1996) Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood 87:1862–1872
Eigenmann DE, Xue G, Kim KS, Moses AV, Hamburger M, Oufir M (2013) Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 10:33
Fontijn RD, Rohlena J, Van Marle J, Pannekoek H, Horrevoets AJ (2006) Limited contribution of claudin-5-dependent tight junction strands to endothelial barrier function. Eur J Cell Biol 85:1131–1144
Paolinelli R, Corada M, Ferrarini L, Devraj K, Arturs C, Czupalla CJ, Rudini N, Maddaluno L et al (2013) Wnt activation of immortalized brain endothelial cells as a tool for generating a standardized model of the blood brain barrier in vitro. PLoS One 8:e70233
Shayan G, Shuler ML, Lee KH (2011) The effect of astrocytes on the induction of barrier properties in aortic endothelial cells. Biotechnol Prog 4:1137–1145
Takeshita Y, Obermeier B, Cotleur A, Sano Y, Kanda T, Ransohoff RM (2014) An in vitro blood-brain barrier model combining shear stress and endothelial cell/astrocyte co-culture. J Neurosci Methods 232:165–172
Ottone C, Parrinello S (2015) Multifaceted control of adult SVZ neurogenesis by the vascular niche. Cell Cycle 14:2222–2225
Goldberg JS, Hirschi KK (2009) Diverse roles of the vasculature within the neural stem cell niche. Regen Med 6:879–897
Hirota S, Clements TP, Tang LK, Morales JE, Lee HS, Oh SP, Rivera GM, Wagner DS et al (2015) Neuropilin 1 balances β8 integrin-activated TGFβ signaling to control sprouting angiogenesis in the brain. Development 24:4363–4373
Javelaud D, Mauviel A (2005) Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene 24:5742–5750
Liebner S, Czupalla CJ, Wolburg H (2011) Current concepts of blood-brain barrier development. Int J Dev Biol 55:467–476
Armendáriz BG, del Masdeu MM, Soriano E, Urena JM, Burgaya F (2014) The diverse roles and multiple forms of focal adhesion kinase in brain. Eur J Neurosci 40:3573–3590
Schlaepfer DD, Mitra SK (2004) Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev 1:92–101
Arnold TD, Niaudet C, Pang MF, Siegenthaler J, Gaengel K, Jung B, Ferrero GM, Mukouyama YS et al (2014) Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain. Development 23:4489–4499
Adams RH, Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8:464–478
Brzozowa M, Wojnicz R, Kowalczyk-Ziomek G, Helewski K (2013) The Notch ligand Delta-like 4 (DLL4) as a target in angiogenesis-based cancer therapy? Contemp Oncol (Pozn) 3:234–237
Aspalter IM, Gordon E, Dubrac A, Ragab A, Narloch J, Vizán P, Geudens I, Collins RT et al (2015) Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Commun 6:7264
Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P et al (2011) Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. PNAS 108:2807–2812
Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M et al (2010) Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science 330:985–989
Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P (2009) Transforming growth factor-beta 1 (TGF-β1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol 219(2):449–458
Özkan A, Biçer A, Avşar T, Şeker A, Toktaş ZO, Bozkurt SU, Başak AN, Kılıç T (2012) Temporal expression analysis of angiogenesis-related genes in brain development. Vascular Cell 4:16
Firth SM, Baxter RC (2002) Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 23(6):824–854
Forbes B, McCarthy P, Norton R (2012) Insulin-like growth factor binding proteins: a structural perspective. Front Endocrinol 3:38
Nieto-Estévez V, Defterali Ç, Vicario-Abejón C (2016) IGF-I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front Neurosci 10
Dai J, Peng L, Fan K, Wang H, Wei R, Ji G, Cai J, Lu B et al (2009) Osteopontin iduces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 28:3412–3422
Rabenstein M, Hucklenbroich J, Willuweit A, Ladwig A, Fink GR, Schroeter M, Langen K-J, Rueger MA (2015) Osteopontin mediates survival, proliferation and migration of neural stem cells through the chemokine receptor CXCR4. Stem cell research & therapy 6(1):1
Wang Y, Yan W, Lu X, Qian C, Zhang J, Li P, Shi L, Zhao P et al (2011) Overexpression of Osteopontin induces angiogenesis of endothelial progenitor cells via the avβ3/P13K/AKT/eNOS/NO signaling pathway in glioma cells. Eur J Cell Biol 90(8):642–648
Stettle EM, Galileo DS (2004) Radial glia produce and align the ligand fibronectin during neuronal migration in the developing chick brain. J Comp Neurol 468(3):441–451
Chirco R, Liu XW, Jung KK, Kim HR (2006) Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 25:99–113
Errede M, Girolamo F, Rizzi M, Bertossi M, Roncali L, Virgintino D (2014) The contribution of CXCL12-expressing radial glia cells to neuro-vascular patterning during human cerebral cortex development. Front Neurosci 8:324
Klenotic PA, Zhang C, Lin Z (2016) Emerging roles of CCN proteins in vascular development and pathology. J Cell Commun Signal 3:251–257
Kurundkar AR, Kurundkar D, Rangarajan S, Locy ML, Zhou Y, Liu RM, Zmijewski J, Thannickal VJ (2016) The matricellular protein CCN1 enhances TGF-β1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury. FASEB J 6:2135–2150
Rodriguez-Grande B, Varghese L, Molina-Holgado F, Rajkovic O, Garlanda C, Denes A, Pinteaux E (2015) Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J Neuroinflammation 12:15
Savarin-Vuaillat C, Ransohoff RM (2007) Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics 4:590–601
Thirumangalakudi L, Samany PG, Owoso A, Wiskar B, Grammas P (2006) Angiogenic proteins are expressed by brain blood vessels in Alzheimer’s disease. J Alzheimers Dis 1:111–118
Thomas M, Augustin HG (2009) The role of the angiopoietins in vascular morphogenesis. Angiogenesis 2:125–137
Yamanaka R, Tanaka R (2004) Gene therapy of brain tumor with endostatin. Drugs Today (Barc) 11:931–934
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C et al (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161:1163–1177
Kostourou V, Papalazarou V (2014) Non-collagenous ECM proteins in blood vessel morphogenesis and cancer. Biochem Biophys Acta 1840:2403–2413
Gomes FCA, de Sousa VO, Romão L (2005) Emerging roles for TGF-β1 in nervous system development. Int J Dev Neurosci 23:413–424
Acknowledgements
We thank Marcelo Meloni and Adiel Batista do Nascimento for technical assistance. We also thank Dr. Dennis Grab from The Johns Hopkins University that kindly provided us with the HBMECs.
Author information
Authors and Affiliations
Contributions
M.S. and D.F. performed MBEC isolation, morphological and functional characterization, RT-PCR and RT-qPCR, Matrigel assays, RG-EC interaction experiments, and Proteome analysis. D.G. produced migration, FAK, Dll4, and TGF-β1 signaling analysis in MBEC, and VEGF expression data on RG cells. F.C.A.G. contributed to data analysis, interpretation, and paper writing. J.S. performed in vivo IUE and IV experiments and data analysis, analyzed all the data, designed the entire project and experimental approaches, and wrote the paper.
Corresponding author
Ethics declarations
All animal protocols were approved by the Animal Research Committee of the Federal University of Rio de Janeiro (DAHEICB024 and CEUA 041/14).
Competing Interests
The authors declare that they have no conflict of interest.
Funding
This work was supported by grants from: Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (J.S.; F.C.A.G.) and Conselho Nacional para o Desenvolvimento Científico e Tecnológico (J.S.; F.C.A.G.; S.M.; D.F.).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Siqueira, M., Francis, D., Gisbert, D. et al. Radial Glia Cells Control Angiogenesis in the Developing Cerebral Cortex Through TGF-β1 Signaling. Mol Neurobiol 55, 3660–3675 (2018). https://doi.org/10.1007/s12035-017-0557-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0557-8