Abstract
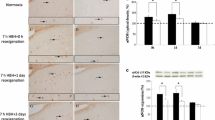
The perinatal brainstem is known to be very vulnerable to hypoxic–ischemic events which can lead to deafness, swallowing dysfunction, and defective respiratory control. The aim of the present work was to evaluate the potential neuroprotective effects of nicotine, melatonin, resveratrol, and docosahexaenoic acid on the expression of a panel of genes in the brainstem following hypoxic–ischemic damage. Quantitative PCR was used to examine gene expression 3 and 12 h after the damage, and immunohistochemistry was employed to evaluate neurons, astrocytes, and synaptic vesicles 24 h post insult. We found that the expression of some immediate-early genes, as well as that of inflammatory genes TNF-α, COX2, and caspase 3, was upregulated in response to the insult. Twenty-four hours after the damage, the percentage of NeuN and synaptophysin immunolabeled cells was found to be reduced while GFAP expression was upregulated. No differences were observed in ROS gene expression following treatments.











Similar content being viewed by others

References
Bellot B, Peyronnet-Roux J, Gire C, Simeoni U, Vinay L, Viemari JC (2014) Deficits of brainstem and spinal cord functions after neonatal hypoxia-ischemia in mice. Pediatr Res 75:723–730
de Vries LS, Jongmans MJ (2010) Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 95:F220–F224
Anderson P, Doyle LW, Victorian Infant Collaborative Study Group. (2003) Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA 289:3264–3272
Marlow N, Rose AS, Rands CE, Draper ES (2005) Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed 90:F380–F387
Martinez-Biarge M, Diez-Sebastian J, Kapellou O, Gindner D, Allsop JM, Rutherford MA, Cowan FM (2011) Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology 76:2055–2061
Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM et al (2012) Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 366:2085–2092
Alonso-Alconada D, Alvarez A, Lacalle J, Hilario E (2012) Histological study of the protective effect of melatonin on neural cells after neonatal hypoxia-ischemia. Histol Histopathol 27:771–783
Johnston MV (2001) Excitotoxicity in neonatal hypoxia. Ment Retard Dev Disabil Res Rev 7:229–234
Panigrahy A, White WF, Rava LA, Kinney HC (1995) Developmental changes in [3H]kainate binding in human brainstem sites vulnerable to perinatal hypoxia-ischemia. Neuroscience 67:441–454
Reinebrant HE, Wixey JA, Buller KM (2012) Disruption of raphé serotonergic neural projections to the cortex: a potential pathway contributing to remote loss of brainstem neurons following neonatal hypoxic-ischemic brain injury. Eur J Neurosci 36:3483–3491
Tang YP, Murata Y, Nagaya T, Noda Y, Seo H, Nabeshima T (1997) NGFI-B, c-fos, and c-jun mRNA expression in mouse brain after acute carbon monoxide intoxication. J Cereb Blood Flow Metab 17:771–780
Chen HH, Liu HM (1996) A new fluorescent histological marker for ischemic neurons, EA 50: correlated with Fos and Jun/AP-1 immunoreactivity. Histochem Cell Biol 105:375–382
Li GC, Li L, Liu RY, Rehman M, Lee WM (1992) Heat shock protein hsp70 protects cells from thermal stress even after deletion of its ATP-binding domain. Proc Natl Acad Sci U S A 89:2036–2040
Kim H, Huh PW, Kim C, Kim YJ, Park EM, Park YM (2001) Cerebral activation and distribution of inducible hsp110 and hsp70 mRNAs following focal ischemia in rat. Toxicology 167:135–144
Tyree MM, Dalgard C, O’Neill JT (2006) Impact of room air resuscitation on early growth response gene-1 in a neonatal piglet model of cerebral hypoxic ischemia. Pediatr Res 59:423–427
Zhang YM, Shi GG, Tang Z, Zheng JH, Li WQ, Guo FX, Jia QY (2006) Effects of N-n-butyl haloperidol iodide on myocardial ischemia/reperfusion injury and Egr-1 expression in rat. Acta Biochim Biophys Sin 38:435–441
Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W (2000) Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol 156:965–976
Schalch P, Patejunas G, Retuerto M, Sarateanu S, Milbrandt J, Thakker G, Kim D, Carbray J et al (2004) Homozygous deletion of early growth response 1 gene and critical limb ischemia after vascular ligation in mice: evidence for a central role in vascular homeostasis. J Thorac Cardiovasc Surg 128:595–601
Kanngiesser M, Mair N, Lim HY, Zschiebsch K, Blees J, Haussler A, Brune B, Ferreiros N et al (2014) Hypoxia-inducible factor 1 regulates heat and cold pain sensitivity and persistence. Antioxid Redox Signal 20:2555–2571
Matsuda T, Abe T, Wu JL, Fujiki M, Kobayashi H (2005) Hypoxia-inducible factor-1alpha DNA induced angiogenesis in a rat cerebral ischemia model. Neurol Res 27:503–508
Hilario E, Rey-Santano MC, Goni-de-Cerio F, Alvarez FJ, Gastiasoro E, Mielgo VE, Caballero A, Valls-i-Soler A et al (2005) Cerebral blood flow and morphological changes after hypoxic-ischaemic injury in preterm lambs. Acta Paediatr 94:903–911
Yang Q, Wu X, Sun J, Cui J, Li L (2014) Epigenetic features induced by ischemia-hypoxia in cultured rat astrocytes. Mol Neurobiol 53(1):436–445. doi:10.1007/s12035-014-9027-8
Pekny M, Wilhelmsson U, Pekna M (2014) The dual role of astrocyte activation and reactive gliosis. Neurosci Lett 565:30–38
Cojocaru IM, Cojocaru M, Tanasescu R, Iliescu I, Dumitrescu L, Silosi I (2009) Expression of IL-6 activity in patients with acute ischemic stroke. Rom J Intern Med 47:393–396
Kalay S, Oztekin O, Tezel G, Aldemir H, Sahin E, Koksoy S, Akcakus M, Oygur N (2013) The effects of intraperitoneal pentoxifylline treatment in rat pups with hypoxic-ischemic encephalopathy. Pediatr Neurol 49:319–323
Li SJ, Liu W, Wang JL, Zhang Y, Zhao DJ, Wang TJ, Li YY (2014) The role of TNF-alpha, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur Rev Med Pharmacol Sci 18:905–909
Bradl M, Lassmann H (2010) Oligodendrocytes: biology and pathology. Acta Neuropathol 119:37–53
Ritter J, Schmitz T, Chew LJ, Buhrer C, Mobius W, Zonouzi M, Gallo V (2013) Neonatal hyperoxia exposure disrupts axon-oligodendrocyte integrity in the subcortical white matter. J Neurosci 33:8990–9002
Schmitz T, Ritter J, Mueller S, Felderhoff-Mueser U, Chew LJ, Gallo V (2011) Cellular changes underlying hyperoxia-induced delay of white matter development. J Neurosci 31:4327–4344
Volpe JJ (2009) The encephalopathy of prematurity—brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol 16:167–178
Tsai YW, Yang YR, Sun SH, Liang KC, Wang RY (2013) Post ischemia intermittent hypoxia induces hippocampal neurogenesis and synaptic alterations and alleviates long-term memory impairment. J Cereb Blood Flow Metab 33:764–773
Guaiquil VH, Golde DW, Beckles DL, Mascareno EJ, Siddiqui MA (2004) Vitamin C inhibits hypoxia-induced damage and apoptotic signaling pathways in cardiomyocytes and ischemic hearts. Free Radic Biol Med 37:1419–1429
Li Q, Huang XJ, He W, Ding J, Jia JT, Fu G, Wang HX, Guo LJ (2009) Neuroprotective potential of fasudil mesylate in brain ischemia-reperfusion injury of rats. Cell Mol Neurobiol 29:169–180
McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM (2006) Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem 281:24171–24181
Alonso-Alconada D, Hilario E, Alvarez FJ, Alvarez A (2012) Apoptotic cell death correlates with ROS overproduction and early cytokine expression after hypoxia-ischemia in fetal lambs. Reprod Sci 19:754–763
Hejmadi MV, Dajas-Bailador F, Barns SM, Jones B, Wonnacott S (2003) Neuroprotection by nicotine against hypoxia-induced apoptosis in cortical cultures involves activation of multiple nicotinic acetylcholine receptor subtypes. Mol Cell Neurosci 24:779–786
Cilio MR, Ferriero DM (2010) Synergistic neuroprotective therapies with hypothermia. Semin Fetal Neonatal Med 15:293–298
Huang SS, Tsai MC, Chih CL, Hung LM, Tsai SK (2001) Resveratrol reduction of infarct size in Long-Evans rats subjected to focal cerebral ischemia. Life Sci 69:1057–1065
Pan HC, Kao TK, Ou YC, Yang DY, Yen YJ, Wang CC, Chuang YH, Liao SL et al (2009) Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J Nutr Biochem 20:715–725
Shimazawa M, Nakajima Y, Mashima Y, Hara H (2009) Docosahexaenoic acid (DHA) has neuroprotective effects against oxidative stress in retinal ganglion cells. Brain Res 1251:269–275
Revuelta M, Arteaga O, Montalvo H, Alvarez A, Hilario E, Martinez-Ibarguen A (2015) Antioxidant treatments recover the alteration of auditory-evoked potentials and reduce morphological damage in the inferior colliculus after perinatal asphyxia in rat. Brain Pathol. doi:10.1111/bpa.12272
Rice JE III, Vannucci RC, Brierley JB (1981) The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9:131–141
Chen Y, Nie H, Tian L, Tong L, Yang L, Lao N, Dong H, Sang H et al (2013) Nicotine-induced neuroprotection against ischemic injury involves activation of endocannabinoid system in rats. Neurochem Res 38:364–370
Carloni S, Perrone S, Buonocore G, Longini M, Proietti F, Balduini W (2008) Melatonin protects from the long-term consequences of a neonatal hypoxic-ischemic brain injury in rats. J Pineal Res 44:157–164
West T, Atzeva M, Holtzman DM (2007) Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev Neurosci 29:363–372
Berman DR, Mozurkewich E, Liu Y, Barks J (2009) Docosahexaenoic acid pretreatment confers neuroprotection in a rat model of perinatal cerebral hypoxia-ischemia. Am J Obstet Gynecol 200:305.e1–305.e6
Yang QK, Xiong JX, Yao ZX (2013) Neuron-NG2 cell synapses: novel functions for regulating NG2 cell proliferation and differentiation. Biomed Res Int 2013:402843
Orioli D, Colaluca IN, Stefanini M, Riva S, Dotti CG, Peverali FA (2006) Rac3-induced neuritogenesis requires binding to Neurabin I. Mol Biol Cell 17:2391–2400
Benitez SG, Castro AE, Patterson SI, Munoz EM, Seltzer AM (2014) Hypoxic preconditioning differentially affects GABAergic and glutamatergic neuronal cells in the injured cerebellum of the neonatal rat. PLoS One 9, e102056
Tagliaferro P, Javier RA, Onaivi ES, Evrard SG, Lujilde J, Brusco A (2006) Neuronal cytoskeleton and synaptic densities are altered after a chronic treatment with the cannabinoid receptor agonist WIN 55,212-2. Brain Res 1085:163–176
Na JI, Na JY, Choi WY, Lee MC, Park MS, Choi KH, Lee JK, Kim KT et al (2015) The HIF-1 inhibitor YC-1 decreases reactive astrocyte formation in a rodent ischemia model. Am J Transl Res 7:751–760
Alvarez-Diaz A, Hilario E, de Cerio FG, Valls-i-Soler A, Alvarez-Diaz FJ (2007) Hypoxic-ischemic injury in the immature brain—key vascular and cellular players. Neonatology 92:227–235
Donega V, Nijboer CH, van Tilborg G, Dijkhuizen RM, Kavelaars A, Heijnen CJ (2014) Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp Neurol 261:53–64
Xiong M, Yang Y, Chen GQ, Zhou WH (2009) Post-ischemic hypothermia for 24h in P7 rats rescues hippocampal neuron: association with decreased astrocyte activation and inflammatory cytokine expression. Brain Res Bull 79:351–357
Tuor UI, Hudzik TJ, Malisza K, Sydserff S, Kozlowski P, Del Bigio MR (2001) Long-term deficits following cerebral hypoxia-ischemia in four-week-old rats: correspondence between behavioral, histological, and magnetic resonance imaging assessments. Exp Neurol 167:272–281
Zins K, Pomyje J, Hofer E, Abraham D, Lucas T, Aharinejad S (2014) Egr-1 upregulates Siva-1 expression and induces cardiac fibroblast apoptosis. Int J Mol Sci 15:1538–1553
Okada M, Yan SF, Pinsky DJ (2002) Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) activation suppresses ischemic induction of Egr-1 and its inflammatory gene targets. FASEB J 16:1861–1868
Yan SF, Fujita T, Lu J, Okada K, Shan ZY, Mackman N, Pinsky DJ, Stern DM (2000) Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med 6:1355–1361
Wu H, Lei S, Yuan J, Liu X, Zhang D, Gu X, Zhang L, Xia Z (2013) Ischemic postconditioning downregulates Egr-1 expression and attenuates postischemic pulmonary inflammatory cytokine release and tissue injury in rats. J Surg Res 181:204–212
Wang HD, Fukuda T, Suzuki T, Hashimoto K, Liou SY, Momoi T, Kosaka T, Yamamoto K et al (1999) Differential effects of Bcl-2 overexpression on hippocampal CA1 neurons and dentate granule cells following hypoxic ischemia in adult mice. J Neurosci Res 57:1–12
Erickson JT, Millhorn DE (1994) Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol 348:161–182
Goetzenich A, Hatam N, Preuss S, Moza A, Bleilevens C, Roehl AB, Autschbach R, Bernhagen J et al (2014) The role of hypoxia-inducible factor-1alpha and vascular endothelial growth factor in late-phase preconditioning with xenon, isoflurane and levosimendan in rat cardiomyocytes. Interact Cardiovasc Thorac Surg 18:321–328
Pascual O, Ben AS, Rostaing P, Triller A, Bessis A (2012) Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A 109:E197–E205
Olivetto E, Simoni E, Guaran V, Astolfi L, Martini A (2015) Sensorineural hearing loss and ischemic injury: development of animal models to assess vascular and oxidative effects. Hear Res 327:58–68
Fujimoto M, Nakai A (2010) The heat shock factor family and adaptation to proteotoxic stress. FEBS J 277:4112–4125
Islam A, Abraham P, Hapner CD, Andrews-Shigaki B, Deuster P, Chen Y (2013) Heat exposure induces tissue stress in heat-intolerant, but not heat-tolerant, mice. Stress 16:244–253
Sun X, Crawford R, Liu C, Luo T, Hu B (2015) Development-dependent regulation of molecular chaperones after hypoxia-ischemia. Neurobiol Dis 82:123–131
Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA (2003) VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111:1843–1851
Kovacs M, Akiskal HS, Gatsonis C, Parrone PL (1994) Childhood-onset dysthymic disorder. Clinical features and prospective naturalistic outcome. Arch Gen Psychiatry 51:365–374
Tarras SL, Diebel LN, Liberati DM, Ginnebaugh K (2013) Pharmacologic stimulation of the nicotinic anti-inflammatory pathway modulates gut and lung injury after hypoxia-reoxygenation injury. Surgery 154:841–847, discussion 847–8
Ma X, Jia Y, Zu S, Li R, Jia Y, Zhao Y, Xiao D, Dang N et al (2014) Alpha5 nicotinic acetylcholine receptor mediates nicotine-induced HIF-1alpha and VEGF expression in non-small cell lung cancer. Toxicol Appl Pharmacol 278:172–179
Acuna-Castroviejo D, Martin M, Macias M, Escames G, Leon J, Khaldy H, Reiter RJ (2001) Melatonin, mitochondria, and cellular bioenergetics. J Pineal Res 30:65–74
Hassell KJ, Ezzati M, Alonso-Alconada D, Hausenloy DJ, Robertson NJ (2015) New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch Dis Child Fetal Neonatal Ed 100(6):F541–F552. doi:10.1136/archdischild-2014-306284
Rossler OG, Glatzel D, Thiel G (2015) Resveratrol upregulates Egr-1 expression and activity involving extracellular signal-regulated protein kinase and ternary complex factors. Exp Cell Res 332:116–127
Yazir Y, Utkan T, Gacar N, Aricioglu F (2015) Resveratrol exerts anti-inflammatory and neuroprotective effects to prevent memory deficits in rats exposed to chronic unpredictable mild stress. Physiol Behav 138:297–304
Simao F, Matte A, Pagnussat AS, Netto CA, Salbego CG (2012) Resveratrol preconditioning modulates inflammatory response in the rat hippocampus following global cerebral ischemia. Neurochem Int 61:659–665
Velten M, Britt RD Jr, Heyob KM, Tipple TE, Rogers LK (2014) Maternal dietary docosahexaenoic acid supplementation attenuates fetal growth restriction and enhances pulmonary function in a newborn mouse model of perinatal inflammation. J Nutr 144:258–266
Acknowledgments
This work was supported by a grant from the Basque Country Government (IT773/13).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experimental procedures received previous approval from the Animal Welfare Committee of the University of the Basque Country (UPV/EHU) and complied with the National Institutes of Health guidelines for the care and use of laboratory animals and the European Communities Directive 86/609/EEC regulating animal research.
Rights and permissions
About this article
Cite this article
Revuelta, M., Arteaga, O., Alvarez, A. et al. Characterization of Gene Expression in the Rat Brainstem After Neonatal Hypoxic–Ischemic Injury and Antioxidant Treatment. Mol Neurobiol 54, 1129–1143 (2017). https://doi.org/10.1007/s12035-016-9724-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9724-6