Abstract
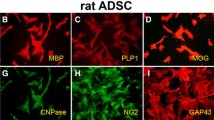
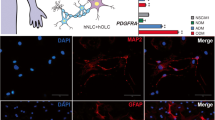
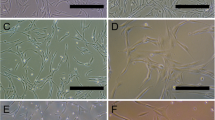
Aquaporins (AQPs) are 13 integral membrane proteins that provide selective pores for the rapid movement of water and other uncharged solutes, across cell membranes. Recently, AQPs have been focused for their role in production, circulation, and homeostasis of the cerebrospinal fluid and their importance in several human diseases is becoming clear. This study investigated the time course (0, 14, and 28 days) of AQP1, 4, 7, 8, and 9 during the neural differentiation of human mesenchymal stem cells (MSCs) from adipose tissue (AT). For this purpose, two different media, enriched with serum or B-27 and N1 supplements, were applied to give a stimulus toward neural lineage. After 14 days, the cells were cultured with neuronal or glial differentiating medium for further 14 days. The results confirmed that AT-MSCs could be differentiated into neurons, astrocytes, and oligodendrocytes, expressing not only the typical neural markers but also specific AQPs depending on differentiated cell type. Our data demonstrated that at 28 days, AT-MSCs express only AQP1; astrocytes AQP1, 4, and 7; oligodendrocytes AQP1, 4, and 8; and finally neurons AQP1 and 7. This study provides fundamental insight into the biology of the mesenchymal stem cells and it suggests that AQPs can be potential neural markers.







Similar content being viewed by others
References
Hill AE, Schachar-Hill B, Schachar-Hill Y (2004) What are aquaporins for? J Membrane Biol 197:1–32. doi:10.1007/s00232-003-0639-6
Zeuthen T, Macaulay N (2002) Passive water transport in biological pores. Int Rev Cytol 215:203–230
Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A 88:11110–11114
Ishibashi K (2006) Aquaporin superfamily with unusual npa boxes: S-aquaporins (superfamily, sip-like and subcellular-aquaporins). Cell Mol Biol 52:20–27. doi:10.1016/j.bbamem.2006.02.024
King LS, Konzo D, Agre P (2004) From structure to disease: the evolving tale of aquaporin biology. Nat Rev Cell Biol 5:687–698. doi:10.1038/nrm1469
Papadopoulos MC, Saadoun S, Verkman AS (2008) Aquaporins and cell migration. Pflugers Arch 456:693–700. doi:10.1007/s00424-007-0357-5
Hoque MO, Soria JC, Woo J et al (2006) Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am J Pathol 168:1345–1353. doi:10.2353/ajpath.2006.050596
Jablonski E, Webb A, Hughes FM (2004) Water movement during apoptosis: a role for aquaporins in the apoptotic volume decrease (AVD). Adv Exp Med Biol 559:179–188
Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG (2001) Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem 276:23413–23420. doi:10.1074/jbc.M008760200
Borsani E (2010) Aquaporins in sensory and pain transmission. Curr Neuropharmacol 8:122–127. doi:10.2174/157015910791233187
Lee WK, Thévenod F (2006) A role for mitochondrial aquaporins in cellular life-and-death decisions? Am J Physiol Cell Physiol 291:C195–C202. doi:10.1152/ajpcell.00641.2005
Cho SJ, Sattar AK, Jeong EH, Satchi M, Cho JA, Dash S, Mayes MS, Stromer MH et al (2002) Aquaporin 1 regulates GTP-induced rapid gating of water in secretory vesicles. Proc Natl Acad Sci U S A 99:4720–4724. doi:10.1073/pnas.072083499
Zhu N, Feng X, He C, Gao H, Yang L, Ma Q, Guo L, Qiao Y et al (2011) Defective macrophage function in aquaporin-3 deficiency. FASEB J 25:4233–4239. doi:10.1096/fj.11-182808
Badaut J, Lasbennes F, Magistretti PJ, Regli L (2002) Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab 22:367–378. doi:10.1097/00004647-200204000-00001
Tait MJ, Saadoum S, Bell BA, Popadopoulos M (2008) Water movements in the brain: the role of aquaporins. Trends Neurosci 31:37–43. doi:10.1016/j.tins.2007.11.003
Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT (2005) Reduced cerebrospinal fluid production and intracranical pressure in mice lacking choroid plexus water channel aquaporin-1. FASEB J 19:76–78. doi:10.1096/fj.04-1711fje
La Porta CA, Gena P, Gritti A, Fascio U, Svelto M, Calamita G (2006) Adult murine CNS stem cells express aquaporin channels. Biol Cell 98:89–94. doi:10.1042/BC20040153
Avril-Delplanque A, Casal I, Castillon N, Hinnrasky J, Puchelle E, Peault B (2005) Aquaporin-3 expression in human fetal airway epithelial progenitor cells. Stem Cells 23:992–1001. doi:10.1634/stemcells.2004-0197
Cavazzin C, Ferrari D, Facchetti F, Russignan A, Vescovi AL, La Porta CA, Gritti A (2006) Unique expression and localization of aquaporin-4 and aquaporin-9 in murine and human neural stem cells and in their glial progeny. Glia 53:167–181. doi:10.1002/glia.20256
Kong H, Fan Y, Xie J, Ding J, Sha L, Shi X, Sun X, Hu G (2008) AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J Cell Sci 211:4029–4036. doi:10.1242/jcs.035758
Yi F, Khan M, Gao H, Hao F, Sun M, Zhong L, Lu C, Feng X et al (2012) Increased differentiation capacity of bone marrow-derived mesenchymal stem cells in aquaporin-5 deficiency. Stem Cells Dev 21:2495–2507. doi:10.1089/scd.2011.0597
Zelenina M, Zelenin S, Aperia A (2005) A water channels (aquaporins) and their role for postnatal adaptation. Pediatr Res 57:47R–53R. doi:10.1203/01.PDR.0000159572.79074.0B
Ashjian PH, Elbarbary AS, Edmonds B, De Ugarte D, Zhu M, Zuk PA, Lorenz HP, Benhaim P et al (2003) In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg 111:1922–1931. doi:10.1097/01.PRS.0000055043.62589.05
Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V et al (2003) Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum 48:418–429. doi:10.1002/art.10767
Guilak F, Estes BT, Diekman BO, Moutos FT, Gimble JM (2010) Multipotent adult stem cells from adipose tissue for muscularskeletal tissue engineering. Clin Orthop Relat Res 468:2530–2540. doi:10.1007/s11999-010-1410-9
Musumeci G, Lo Furno D, Loreto C, Giuffrida R, Caggia S, Leonardi R, Cardile V (2011) Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp Biol Med 236:1333–1341. doi:10.1258/ebm.2011.011183
Musumeci G, Mobasheri A, Trovato FM, Szychlinska MA, Graziano AC, Lo Furno D, Avola R, Mangano S et al (2014) Biosynthesis of collagen I, II, RUNX2 and lubricin at different timepoints of chondrogenic differentiation in a 3D in vitro model of human mesenchymal stem cells derived from adipose tissue. Acta Histochem 116:1407–1417. doi:10.1016/j.acthis.2014.09.008
Lo Furno D, Pellitteri R, Graziano ACE, Giuffrida R, Vancheri C, Gili E, Cardile V (2013) Differentiation of human adipose stem cells into neural phenotype by neuroblastoma- or olfactory ensheathing cells-conditioned medium. J Cell Physiol 228:2109–2118. doi:10.1002/jcp.24386
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295. doi:10.1091/mbc.E02-02-0105
Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595
Gilyarov AV (2008) Nestin in central nervous system cells. Neurosci Behav Physiol 38:165–169. doi:10.1007/s11055-008-0025-z
Rex M, Orme A, Uwanogho D, Tointon K, Wigmore PM, Sharpe PT, Scotting PJ (1997) Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn 209:323–332. doi:10.1002/(SICI)1097-0177(199707)209:3<323::AID-AJA7>3.0.CO;2-K
Graham V, Khudyakov J, Ellis P, Pevny L (2003) SOX2 functions to maintain neural progenitor. Identity 39:749–765
Bylund M, Andersson E, Novitch BG, Muhr J (2003) Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci 6:1162–1168. doi:10.1038/nn1131
Hutton SR, Pevny LH (2011) SOX2 expression levels distinguish between neural progenitor populations of the developing dorsal telencephalon. Dev Biol 352:40–47. doi:10.1016/j.ydbio.2011.01.015
Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR et al (2004) Nestin expression—a property of multi-lineage progenitor cells? Cell Mol Life Sci 61:2510–2522. doi:10.1007/s00018-004-4144-6
Copp AJ, Greene NDE (2010) Genetics and development of neural tube defects. J Pathol 220:217–230. doi:10.1002/path.2643
Ferroni L, Gardin C, Tocco I, Epis R, Casadei A, Vindigni V, Mucci G, Zavan B (2013) Potential for neural differentiation of mesenchymal stem cells. Adv Biochem Eng Biotechnol 129:89–115. doi:10.1007/10_2012_152
Li YC, Lin YC, Young TH (2012) Combination of media, biomaterials and extracellular matrix proteins to enhance the differentiation of neural stem/precursor cells into neurons. Acta Biomater 8:3035–3048. doi:10.1016/j.actbio.2012.04.036
Xu L, Ryu J, Hiel H, Menon A, Aggarwal A, Rha E, Mahairaki V, Cummings BJ et al (2015) Transplantation of human oligodendrocyte progenitor cells in an animal model of diffuse traumatic axonal injury: survival and differentiation. Stem Cell Res Ther 6:93. doi:10.1186/s13287-015-0087-0
Wen H, Nagelhus EA, Amiry-Moghaddam M, Agre P, Ottersen OP, Nielsen S (1999) Ontogeny of water transport in rat brain: postnatal expression of the aquaporin-4 water channel. Eur J Neurosci 11:935–945
Nielsen S, Smith BL, Christensen EI, Agre P (1993) Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A 90:7275–7279
Benga O, Huber VJ (2012) Brain water channel proteins in health and disease. Mol Asp Med 33:562–578. doi:10.1016/j.mam.2012.03.008
Mobasheri A, Marples D (2004) Expression of the AQP-1 water channel in normal human tissues: a semi-quantitative study using tissue mircroarray technology. Am J Phys 286:C529–C537. doi:10.1152/ajpcell.00408.2003
Badaut J, Regli L (2004) Distribution and possible roles of AQP9 in brain. Neurosci 129:971–981. doi:10.1016/j.neuroscience.2004.06.035
Magistretti PJ, Pellerin L, Rothman DL, Shulman RG (1999) Energy on demand. Science 283:496–497
Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P (1997) Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci U S A 91:13052–13056
Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP (1997) Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17:171–180
Solenov E, Watanabe H, Manley GT, Verkman AS (2003) Seven-fold reduced osmotic water permeability in primary astrocyte cultures from aquaporin-4 deficient mice measured by a calcein quenching method. Am J Physiol Cell Physiol 286:426–432. doi:10.1152/ajpcell.00298.2003
Horio Y (2001) Potassium channels of glial cells: distribution and function. Jpn J Pharmacol 87:1–6
Ferri D, Mazzone A, Liquori GE, Cassano G, Svelto M, Calamita G (2003) Ontogeny, distribution, and possible functional implications of an unusual aquaporin, AQP8, in mouse liver. Hepatology 38:947–957. doi:10.1053/jhep.2003.50397
Calamita G, Ferri D, Gena P, Liquori GE, Cavalier A, Thomas D, Svelto M (2005) The inner mitochondrial membrane has aquaporin-8 water channels and is highly permeable to water. J Biol Chem 280:17149–17153. doi:10.1074/jbc.C400595200
Yamamoto N, Yoneda K, Asai K, Sobue K, Tada T, Fujita Y, Katsuya H, Fujita M et al (2001) Alterations in the expression of the AQP family in cultured rat astrocytes during hypoxia and reoxygenation. Brain Res Mol Brain Res 90:26–38
Acknowledgements
The authors wish to tank Prof. G. Musumeci, University of Catania, Department of Biomedical and Biotechnological Sciences, Section of Anatomy and Histology, and Prof. E. Barbagallo, University of Catania, Department of Drug Sciences, Section of Biochemistry, for having kindly provided AQP1 antibody and lipoaspirates, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Dedicated to the memory of Carmela Spatafora
Rights and permissions
About this article
Cite this article
Avola, R., Graziano, A.C.E., Pannuzzo, G. et al. Human Mesenchymal Stem Cells from Adipose Tissue Differentiated into Neuronal or Glial Phenotype Express Different Aquaporins. Mol Neurobiol 54, 8308–8320 (2017). https://doi.org/10.1007/s12035-016-0312-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0312-6