Abstract
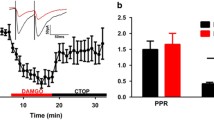
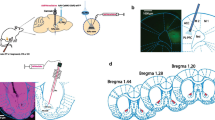
The ventrolateral periaqueductal gray (vlPAG) is an important brain area, in which 5-HTergic neurons play key roles in descending pain modulation. It has been proposed that opioid peptides within the vlPAG can excite the 5-HTergic neurons by alleviating tonic inhibition from GABAergic neurons, the so-called disinhibitory effect. However, no direct morphological evidence has been observed for the micro-circuitry among the opioid peptide-, GABA-, and 5-HT-immunoreactive (ir) profiles nor for the functional involvement of the opioid peptides in the intrinsic properties of GABAergic and 5-HTergic neurons. In the present study, through microscopic observation of triple-immunofluorescence, we firstly identified the circuitry among the endomorphin-1 (EM1, an endogenous ligand for the μ-opioid receptor)-ir terminals and GABA-ir and 5-HT-ir neurons within the rat vlPAG. The synaptic connections of these neurons were further confirmed by electron microscopy. Through the in vitro whole-cell patch-clamp method, we showed that EM1 has strong inhibitory effects on the spiking of GABAergic neurons. However, although the resting membrane potential was hyperpolarized, EM1 actually increased the firing of 5-HTergic neurons. More interestingly, EM1 strongly inhibited the excitatory input to GABAergic neurons, as well as the inhibitory input to 5-HTergic neurons. Finally, behavioral results showed that pretreatment with a GABAA receptor antagonist potentiated the analgesic effect of EM1, while treatment with a GABAA receptor agonist blocked its analgesic effect. In summary, by utilizing morphological and functional methods, we found that the analgesic effect of EM1 is largely dependent on its potent inhibition on the inhibitory inputs to 5-HTergic neurons, which overwhelms EM1’s direct inhibitory effect on 5-HTergic neurons.










Similar content being viewed by others
References
Basbaum AI, Braz JM (2010) Transgenic mouse models for the tracing of “pain” pathways. In: Kruger L, Light AR (eds) Translational pain research: from mouse to man. Frontiers in Neuroscience, Boca Raton
Zhuo M, Gebhart GF (2002) Modulation of noxious and non-noxious spinal mechanical transmission from the rostral medial medulla in the rat. J Neurophysiol 88(6):2928–2941
Zhuo M, Sengupta JN, Gebhart GF (2002) Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol 87(5):2225–2236
Basbaum AI, Fields HL (1984) Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7:309–338
Lau BK, Vaughan CW (2014) Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol 29C:159–164
Barbaro NM (1988) Studies of PAG/PVG stimulation for pain relief in humans. Prog Brain Res 77:165–173
Behbehani MM (1995) Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol 46(6):575–605
Gebhart GF, Sandkuhler J, Thalhammer JG, Zimmermann M (1984) Inhibition in spinal cord of nociceptive information by electrical stimulation and morphine microinjection at identical sites in midbrain of the cat. J Neurophysiol 51(1):75–89
Jacquet YF, Lajtha A (1973) Morphine action at central nervous system sites in rat: analgesia or hyperalgesia depending on site and dose. Science 182(4111):490–492
Jones SL, Gebhart GF (1988) Inhibition of spinal nociceptive transmission from the midbrain, pons and medulla in the rat: activation of descending inhibition by morphine, glutamate and electrical stimulation. Brain Res 460(2):281–296
Lipp J (1991) Possible mechanisms of morphine analgesia. Clin Neuropharmacol 14:131–147
Bodnar RJ (2013) Endogenous opiates and behavior: 2012. Peptides 50:55–95
Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474
Depaulis A, Morgan MM, Liebeskind JC (1987) GABAergic modulation of the analgesic effects of morphine microinjected in the ventral periaqueductal gray matter of the rat. Brain Res 436(2):223–228
Ingram SL, Macey TA, Fossum EN, Morgan MM (2008) Tolerance to repeated morphine administration is associated with increased potency of opioid agonists. Neuropsychopharmacology 33(10):2494–2504
Mehalick ML, Ingram SL, Aicher SA, Morgan MM (2013) Chronic inflammatory pain prevents tolerance to the antinociceptive effect of morphine microinjected into the ventrolateral periaqueductal gray of the rat. J Pain 14(12):1601–1610
Moreau JL, Fields HL (1986) Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res 397(1):37–46
Park C, Kim JH, Yoon BE, Choi EJ, Lee CJ, Shin HS (2010) T-type channels control the opioidergic descending analgesia at the low threshold-spiking GABAergic neurons in the periaqueductal gray. Proc Natl Acad Sci U S A 107(33):14857–14862
Chieng B, Christie MJ (1994) Inhibition by opioids acting on μ-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol 113:303–309
Kishimoto K, Koyama S, Akaike N (2001) Synergistic mu-opioid and 5-HT1A presynaptic inhibition of GABA release in rat periaqueductal gray neurons. Neuropharmacology 41(5):529–538
Ogawa S, Kow LM, Pfaff DW (1994) In vitro electrophysiological characterization of midbrain periaqueductal gray neurons in female rats: responses to GABA- and Met-enkephalin-related agents. Brain Res 666(2):239–249
Morgan MM, Clayton CC (2005) Defensive behaviors evoked from the ventrolateral periaqueductal gray of the rat: comparison of opioid and GABA disinhibition. Behav Brain Res 164(1):61–66
Vaughan CW, Christie MJ (1997) Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol 498:463–472
Vaughan CW, Ingram SL, Connor MA (1997) How opioids inhibit GABA-mediated neurotransmission. Nature 390:611–614
Chen T, Hui R, Wang XL, Zhang T, Dong YX, Li YQ (2008) Origins of endomorphin-immunoreactive fibers and terminals in different columns of the periaqueductal gray in the rat. J Comp Neurol 509(1):72–87
Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE (1999) Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol 405:450–471
Gioia M, Bianchi R (1995) Enkephalin in the caudal PAG of rat: an immunocytochemical electron microscopic study. J Hirnforsch 36(3):421–431
Williams FG, Beitz AJ (1990) Ultrastructural morphometric analysis of enkephalin-immunoreactive terminals in the ventrocaudal periaqueductal gray: analysis of their relationship to periaqueductal gray-raphe magnus projection neurons. Neuroscience 38(2):381–394
Commons KG, Aicher SA, Kow LM, Pfaff DW (2000) Presynaptic and postsynaptic relations of mu-opioid receptors to gamma-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J Comp Neurol 419(4):532–542
Kalyuzhny AE, Wessendorf MW (1998) Relationship of mu- and delta-opioid receptors to GABAergic neurons in the central nervous system, including antinociceptive brainstem circuits. J Comp Neurol 392(4):528–547
Behbehani MM, Jiang MR, Chandler SD, Ennis M (1990) The effect of GABA and its antagonists on midbrain periaqueductal gray neurons in the rat. Pain 40(2):195–204
Reichling DB, Basbaum AI (1990) Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: II. Electron microscopic immunocytochemical evidence of GABAergic control over the projection from the periaqueductal gray to the nucleus raphe magnus in the rat. J Comp Neurol 302(2):378–393
Williams FG, Beitz AJ (1990) Ultrastructural morphometric analysis of GABA-immunoreactive terminals in the ventrocaudal periaqueductal grey: analysis of the relationship of GABA terminals and the GABAA receptor to periaqueductal grey-raphe magnus projection neurons. J Neurocytol 19(5):686–696
Chiou LC, Huang LY (1999) Mechanism underlying increased neuronal activity in the rat ventrolateral periaqueductal grey by a mu-opioid. J Physiol 518(Pt 2):551–559
Fichna J, Janecka A, Costentin J, Do Rego JC (2007) The endomorphin system and its evolving neurophysiological role. Pharmacol Rev 59(1):88–123
Lazarus LH, Okada Y (2012) Engineering endomorphin drugs: state of the art. Expert Opin Ther Pat 22(1):1–14
Hui R, Wang W, Chen T, Lu BC, Li H, Zhang T, Wu SX, Li YQ (2010) Origins of endomorphin-2 immunopositive fibers and terminals in the spinal dorsal horn of the rat. Neuroscience 169:422–430
Pierce TL, Wessendorf MW (2000) Immunocytochemical mapping of endomorphin-2 immunoreactivity in rat brain. J Chem Neuroanat 18:181–207
Tseng LF, Narita M, Suganuma C, Mizoguchi H, Ohsawa M, Nagase H, Kampine JP (2000) Differential antinociceptive effects of endomorphin-1 and endomorphin-2 in the mouse. J Pharmacol Exp Ther 292:576–583
Hao S, Mamiya K, Takahata O, Iwasaki H, Mata M, Fink DJ (2003) Nifedipine potentiates the antinociceptive effect of endomorphin-1 microinjected into the periaqueductal gray in rats. Anesth Analg 96(4):1065–1067
Terashvili M, Wu HE, Leitermann RJ, Sun HS, Clithero ADTL (2005) Differential mechanisms of antianalgesia induced by endomorphin-1 and endomorphin-2 in the ventral periaqueductal gray of the rat. J Pharmacol Exp Ther 312:1257–1265
Ge SN, Li ZH, Tang J, Ma Y, Hioki H, Zhang T, Lu YC, Zhang FX, Mizuno N, Kaneko T, Liu YY, Lung MS, Gao GD, Li JL (2014) Differential expression of VGLUT1 or VGLUT2 in the trigeminothalamic or trigeminocerebellar projection neurons in the rat. Brain Struct Funct 219(1):211–229
Huo FQ, Chen T, Lv BC, Wang J, Zhang T, Qu CL, Li YQ, Tang JS (2009) Synaptic connections between GABAergic elements and serotonergic terminals or projecting neurons in the ventrolateral orbital cortex. Cereb Cortex 19(6):1263–1272
Li JL, Wang D, Kaneko T, Shigemoto R, Nomura S, Mizuno N (2000) Relationship between neurokinin-1 receptor and substance P in the striatum: light and electron microscopic immunohistochemical study in the rat. J Comp Neurol 418:156–163
Huo FQ, Qu CL, Li YQ, Tang JS, Jia H (2008) GABAergic modulation is involved in the ventrolateral orbital cortex 5-HT 1A receptor activation-induced antinociception in the rat. Pain 139(2):398–405
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32(1):77–88
Tillu DV, Gebhart GF, Sluka KA (2008) Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain 136(3):331–339
Chen T, Koga K, Descalzi G, Qiu S, Wang J, Zhang LS, Zhang ZJ, He XB, Qin X, Xu FQ, Hu J, Wei F, Huganir RL, Li YQ, Zhuo M (2014) Postsynaptic potentiation of corticospinal projecting neurons in the anterior cingulate cortex after nerve injury. Mol Pain 10:33
Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M (2010) Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 330(6009):1400–1404
Cao XY, Xu H, Wu LJ, Li XY, Chen T, Zhuo M (2009) Characterization of intrinsic properties of cingulate pyramidal neurons in adult mice after nerve injury. Mol Pain 5:73
Ding YQ, Kaneko T, Nomura S, Mizuno N (1996) Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol 367(3):375–402
Mollereau C, Mouledous L (2000) Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides 21(7):907–917
Moskowitz AS, Goodman RR (1985) Autoradiographic distribution of mu1 and mu2 opioid binding in the mouse central nervous system. Brain Res 360(1–2):117–129
Connor M, Christie MJ (1998) Modulation of Ca2+ channel currents of acutely dissociated rat periaqueductal grey neurons. J Physiol 509(Pt 1):47–58
Ingram SL, Fossum EN, Morgan MM (2007) Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology 32(3):600–606
Wang QP, Nakai Y (1993) Enkephalinergic innervation of GABAergic neurons in the dorsal raphe nucleus of the rat. Brain Res Bull 32(3):315–320
Wang QP, Zadina JE, Guan JL, Shioda S (2002) Morphological studies of the endomorphinergic neurons in the central nervous system. Jpn J Pharmacol 89(3):209–215
Osborne PB, Vaughan CW, Wilson HI, Christie MJ (1996) Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J Physiol 490(Pt 2):383–389
Bagley EE, Chieng BC, Christie MJ, Connor M (2005) Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br J Pharmacol 146(1):68–76
Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH (1998) Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 82:443–468
Beitz AJ (1989) Possible origin of glutamatergic projections to the midbrain periaqueductal gray and deep layer of the superior colliculus of the rat. Brain Res Bull 23(1–2):25–35
Liu ZL, Ma H, Xu RX, Dai YW, Zhang HT, Yao XQ, Yang K (2012) Potassium channels underlie postsynaptic but not presynaptic GABAB receptor-mediated inhibition on ventrolateral periaqueductal gray neurons. Brain Res Bull 88(5):529–533
Acknowledgments
The authors wish to thank Dr. Giannina Descalzi (Department of Neuroscience, Icahn School of Medicine, USA) for carefully reading and constructive suggestions on the article. This work was supported by National Natural Science Foundation of China (31010103909, 81371239 to Y.-Q. Li, 31171068 to J.-Q. Du and 31371126 to T. Chen).
Conflict of Interest
The authors declare that they have no conflicts of interest with any of the work presented in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tao Chen, Jing Li, Ban Feng, Rui Hui and Yu-Lin Dong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, T., Li, J., Feng, B. et al. Mechanism Underlying the Analgesic Effect Exerted by Endomorphin-1 in the rat Ventrolateral Periaqueductal Gray. Mol Neurobiol 53, 2036–2053 (2016). https://doi.org/10.1007/s12035-015-9159-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9159-5