Abstract
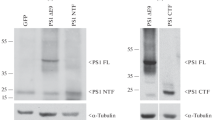
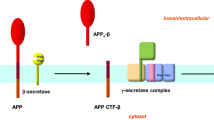
Early-onset familial Alzheimer’s disease (AD) is most commonly associated with the mutations in presenilin-1 (PS1). PS1 is the catalytic component of the γ-secretase complex, which cleaves amyloid precursor protein to produce amyloid-β (Aβ), the major cause of AD. Presenilin enhancer 2 (Pen2) is critical for activating γ-secretase and exporting PS1 from endoplasmic reticulum (ER). Among all the familial AD-linked PS1 mutations, mutations at the G206 amino acid are the most adjacent position to the Pen2 binding site. Here, we characterized the effect of a familial AD-linked PS1 G206D mutation on the PS1-Pen2 interaction and the accompanied alteration in γ-secretase-dependent and -independent functions. We found that the G206D mutation reduced PS1-Pen2 interaction, but did not abolish γ-secretase formation and PS1 endoproteolysis. For γ-secretase-dependent function, the G206D mutation increased Aβ42 production but not Notch cleavage. For γ-secretase-independent function, this mutation disrupted the ER calcium homeostasis but not lysosomal calcium homeostasis and autophagosome maturation. Impaired ER calcium homeostasis may due to the reduced mutant PS1 level in the ER. Although this mutation did not alter the cell survival under stress, both increased Aβ42 ratio and disturbed ER calcium regulation could be the mechanisms underlying the pathogenesis of the familial AD-linked PS1 G206D mutation.






Similar content being viewed by others
References
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344. doi:10.1056/NEJMra0909142
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356. doi:10.1126/science.1072994
Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ (1993) b-Amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A 90:10836–10840. doi:10.1073/pnas.90.22.10836
McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T (2005) Ab42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron 47(2):191–199. doi:10.1016/j.neuron.2005.06.030
Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C (2003) Reconstitution of g-secretase activity. Nat Cell Biol 5(5):486–488. doi:10.1038/ncb960
Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T (2003) The role of presenilin cofactors in the gamma-secretase complex. Nature 422(6930):438–441. doi:10.1038/nature01506
De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R (1999) A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398(6727):518–522. doi:10.1038/19083
De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F (1998) Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391:387–390. doi:10.1038/34910
Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, Baekelandt V, Dressel R, Cupers P, Huylebroeck D, Zwijsen A, Van Leuven F, De Strooper B (1999) Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci U S A 96(21):11872–11877. doi:10.1073/pnas.96.21.11872
Rechards M, Xia W, Oorschot VM, Selkoe DJ, Klumperman J (2003) Presenilin-1 exists in both pre- and post-Golgi compartments and recycles via COPI-coated membranes. Traffic 4(8):553–565. doi:10.1034/j.1600-0854.2003.t01-1-00114.x
Haass C, Kaether C, Thinakaran G, Sisodia S (2012) Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2(5):a006270. doi:10.1101/cshperspect.a006270
Cupers P, Bentahir M, Craessaerts K, Orlans I, Vanderstichele H, Saftig P, De Strooper B, Annaert W (2001) The discrepancy between presenilin subcellular localization and gamma-secretase processing of amyloid precursor protein. J Cell Biol 154(4):731–740. doi:10.1083/jcb.200104045
Fassler M, Zocher M, Klare S, de la Fuente AG, Scheuermann J, Capell A, Haass C, Valkova C, Veerappan A, Schneider D, Kaether C (2010) Masking of transmembrane-based retention signals controls ER export of gamma-secretase. Traffic 11(2):250–258. doi:10.1111/j.1600-0854.2009.01014.x
Kim SH, Sisodia SS (2005) Evidence that the “NF” motif in transmembrane domain 4 of presenilin 1 is critical for binding with PEN-2. J Biol Chem 280(51):41953–41966. doi:10.1074/jbc.M509070200
Watanabe N, Tomita T, Sato C, Kitamura T, Morohashi Y, Iwatsubo T (2005) Pen-2 is incorporated into the gamma-secretase complex through binding to transmembrane domain 4 of presenilin 1. J Biol Chem 280(51):41967–41975. doi:10.1074/jbc.M509066200
Fassler M, Li X, Kaether C (2011) Polar transmembrane-based amino acids in presenilin 1 are involved in endoplasmic reticulum localization, Pen2 protein binding, and gamma-secretase complex stabilization. J Biol Chem 286(44):38390–38396. doi:10.1074/jbc.M111.252429
Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y (2009) gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci 29(41):13042–13052. doi:10.1523/JNEUROSCI. 2362-09.2009
Chavez-Gutierrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, Wiltfang J, Serneels L, Karran E, Gijsen H, Schymkowitz J, Rousseau F, Broersen K, De Strooper B (2012) The mechanism of gamma-Secretase dysfunction in familial Alzheimer disease. EMBO J 31(10):2261–2274. doi:10.1038/emboj.2012.79
Weidemann A, Eggert S, Reinhard FB, Vogel M, Paliga K, Baier G, Masters CL, Beyreuther K, Evin G (2002) A novel epsilon-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with Notch processing. Biochemistry 41(8):2825–2835. doi:10.1021/bi015794o
Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron MM, Teplow DB, Haass C (2001) Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep 2(9):835–841. doi:10.1093/embo-reports/kve180
Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S (1997) Skeletal and CNS defects in presenilin-1-deficient mice. Cell 89:629–639. doi:10.1016/S0092-8674(00)80244-5
Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LH, Sisodia SS (1997) Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 387:288–292. doi:10.1038/387288a0
Shen J, Kelleher RJ 3rd (2007) The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A 104(2):403–409. doi:10.1073/pnas.0608332104
Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee S-F, Hao Y-H, Serneels L, De Strooper B, Yu G, Bezprozvanny I (2006) Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell 126(5):981–993. doi:10.1016/j.cell.2006.06.059
Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141(7):1146–1158. doi:10.1016/j.cell.2010.05.008
Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP (2000) Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem 275(24):18195–18200. doi:10.1074/jbc.m000040200
Stutzmann GE, Caccamo A, LaFerla FM, Parker I (2004) Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci 24(2):508–513. doi:10.1523/JNEUROSCI. 4386-03.2004
Bandara S, Malmersjo S, Meyer T (2013) Regulators of calcium homeostasis identified by inference of kinetic model parameters from live single cells perturbed by siRNA. Sci Signal 6(283):ra56. doi:10.1126/scisignal.2003649
Bezprozvanny I (2013) Presenilins and calcium signaling-systems biology to the rescue. Sci Signal 6(283):pe24. doi:10.1126/scisignal.2004296
Sun S, Zhang H, Liu J, Popugaeva E, Xu NJ, Feske S, White CL 3rd, Bezprozvanny I (2014) Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron 82(1):79–93. doi:10.1016/j.neuron.2014.02.019
Schneider I, Reversé D, Dewachter I, Ris L, Caluwaerts N, Kuipéri C, Gilis M, Geerts H, Kretzschmar H, Godaux E, Moechars D, Van Leuven F, Herms J (2001) Mutant presenilins disturb neuronal calcium homeostasis in the brain of transgenic mice, decreasing the threshold for excitotoxicity and facilitating long-term potentiation. J Biol Chem 276:11539–11544. doi:10.1074/jbc.M010977200
Goussakov I, Miller MB, Stutzmann GE (2010) NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J Neurosci 30(36):12128–12137. doi:10.1523/JNEUROSCI. 2474-10.2010
Wu B, Yamaguchi H, Lai FA, Shen J (2013) Presenilins regulate calcium homeostasis and presynaptic function via ryanodine receptors in hippocampal neurons. Proc Natl Acad Sci U S A 110(37):15091–15096. doi:10.1073/pnas.1304171110
Neely KM, Green KN, LaFerla FM (2011) Presenilin is necessary for efficient proteolysis through the autophagy-lysosome system in a gamma-secretase-independent manner. J Neurosci 31(8):2781–2791. doi:10.1523/JNEUROSCI. 5156-10.2010
Luzio JP, Bright NA, Pryor PR (2007) The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem Soc Trans 35(Pt 5):1088–1091. doi:10.1042/BST0351088
Saftig P, Klumperman J (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10(9):623–635. doi:10.1038/nrm2745
Bezprozvanny I (2012) Presenilins: a novel link between intracellular calcium signaling and lysosomal function? J Cell Biol 198(1):7–10. doi:10.1083/jcb.201206003
Coen K, Flannagan RS, Baron S, Carraro-Lacroix LR, Wang D, Vermeire W, Michiels C, Munck S, Baert V, Sugita S, Wuytack F, Hiesinger PR, Grinstein S, Annaert W (2012) Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J Cell Biol 198(1):23–35. doi:10.1083/jcb.201201076
Neely Kayala KM, Dickinson GD, Minassian A, Walls KC, Green KN, Laferla FM (2012) Presenilin-null cells have altered two-pore calcium channel expression and lysosomal calcium: implications for lysosomal function. Brain Res 1489:8–16. doi:10.1016/j.brainres.2012.10.036
Baki L, Neve RL, Shao Z, Shioi J, Georgakopoulos A, Robakis NK (2008) Wild-type but not FAD mutant presenilin-1 prevents neuronal degeneration by promoting phosphatidylinositol 3-kinase neuroprotective signaling. J Neurosci 28(2):483–490. doi:10.1523/jneurosci. 4067-07.2008
Tanzi R, Bertram L (2005) Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 120:545–555. doi:10.1016/j.cell.2005.02.008
Wu Y-Y, Cheng IH-J, Lee C-C, Chiu M-J, Lee M-J, Chen T-F, Hsu J-L (2011) Clinical phenotype of G206D mutation in the presenilin 1 gene in pathologically confirmed familial Alzheimer’s disease. J Alzheimers Dis 25(1):145–150. doi:10.3233/JAD-2011-102031
Raux G, Guyant-Maréchal L, Martin C, Bou J, Penet C, Brice A, Hannequin D, Frebourg T, Campion D (2005) Molecular diagnosis of autosomal dominant early onset Alzheimer’s disease: an update. J Med Genet 42(10):793–795. doi:10.1136/jmg.2005.033456
Kuo LH, Hu MK, Hsu WM, Tung YT, Wang BJ, Tsai WW, Yen CT, Liao YF (2008) Tumor necrosis factor-alpha-elicited stimulation of gamma-secretase is mediated by c-Jun N-terminal kinase-dependent phosphorylation of presenilin and nicastrin. Mol Biol Cell 19(10):4201–4212. doi:10.1091/mbc.E07-09-0987
Tung YT, Wang BJ, Hsu WM, Hu MK, Her GM, Huang WP, Liao YF (2014) Presenilin-1 regulates the expression of p62 to govern p62-dependent Tau degradation. Mol Neurobiol 49(1):10–27. doi:10.1007/s12035-013-8482-y
Tung YT, Hsu WM, Lee H, Huang WP, Liao YF (2010) The evolutionarily conserved interaction between LC3 and p62 selectively mediates autophagy-dependent degradation of mutant huntingtin. Cell Mol Neurobiol 30(5):795–806. doi:10.1007/s10571-010-9507-y
Yang LT, Nichols JT, Yao C, Manilay JO, Robey EA, Weinmaster G (2005) Fringe glycosyltransferases differentially modulate Notch1 proteolysis induced by Delta1 and Jagged1. Mol Biol Cell 16(2):927–942. doi:10.1091/mbc.E04-07-0614
Tseng LC, Chen RH (2011) Temporal control of nuclear envelope assembly by phosphorylation of lamin B receptor. Mol Biol Cell 22(18):3306–3317. doi:10.1091/mbc.E11-03-0199
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260(6):3440–3450
Kimura S, Noda T, Yoshimori T (2007) Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3(5):452–460. doi:10.4161/auto.4451
Riccardi C, Nicoletti I (2006) Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1(3):1458–1461. doi:10.1038/nprot.2006.238
Dean PN, Jett JH (1974) Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol 60(2):523–527. doi:10.1083/jcb.60.2.523
Chen F, Gu Y, Hasegawa H, Ruan X, Arawaka S, Fraser P, Westaway D, Mount H, St George-Hyslop P (2002) Presenilin 1 mutations activate gamma 42-secretase but reciprocally inhibit epsilon-secretase cleavage of amyloid precursor protein (APP) and S3-cleavage of notch. J Biol Chem 277(39):36521–36526. doi:10.1074/jbc.M205093200
Moehlmann T, Winkler E, Xia X, Edbauer D, Murrell J, Capell A, Kaether C, Zheng H, Ghetti B, Haass C, Steiner H (2002) Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Abeta 42 production. Proc Natl Acad Sci U S A 99(12):8025–8030. doi:10.1073/pnas.112686799
Schroeter EH, Kisslinger JA, Kopan R (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393(6683):382–386. doi:10.1038/30756
Shilling D, Mak DO, Kang DE, Foskett JK (2012) Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. J Biol Chem 287(14):10933–10944. doi:10.1074/jbc.M111.300491
Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, LaFerla FM (2008) SERCA pump activity is physiologically regulated by presenilin and regulates amyloid β production. J Cell Biol 181(7):1107–1116. doi:10.1083/jcb.200706171
Kalies KU, Rapoport TA, Hartmann E (1998) The beta subunit of the Sec61 complex facilitates cotranslational protein transport and interacts with the signal peptidase during translocation. J Cell Biol 141(4):887–894. doi:10.1083/jcb.141.4.887
Petanceska SS, Seeger M, Checler F, Gandy S (2000) Mutant presenilin 1 increases the levels of Alzheimer amyloid beta-peptide Abeta42 in late compartments of the constitutive secretory pathway. J Neurochem 74(5):1878–1884. doi:10.1046/j.1471-4159.2000.0741878.x
Gorvel JP, Chavrier P, Zerial M, Gruenberg J (1991) rab5 controls early endosome fusion in vitro. Cell 64(5):915–925. doi:10.1016/0092-8674(91)90316-q
Mohmmad Abdul H, Sultana R, Keller JN, St Clair DK, Markesbery WR, Butterfield DA (2006) Mutations in amyloid precursor protein and presenilin-1 genes increase the basal oxidative stress in murine neuronal cells and lead to increased sensitivity to oxidative stress mediated by amyloid beta-peptide (1–42), HO and kainic acid: implications for Alzheimer’s disease. J Neurochem 96(5):1322–1335. doi:10.1111/j.1471-4159.2005.03647.x
Nakajima M, Miura M, Aosaki T, Shirasawa T (2001) Deficiency of presenilin-1 increases calcium-dependent vulnerability of neurons to oxidative stress in vitro. J Neurochem 78(4):807–814. doi:10.1046/j.1471-4159.2001.00478.x
Lizard G, Miguet C, Gueldry S, Monier S, Gambert P (1997) Flow cytometry measurement of DNA fragmentation in the course of cell death via apoptosis. New techniques for evaluation of DNA status for the pathologist. Ann Pathol 17(1):61–66. doi: AP-03-1997-17-1-0242-6498-101019-ART85
Kaether C, Capell A, Edbauer D, Winkler E, Novak B, Steiner H, Haass C (2004) The presenilin C-terminus is required for ER-retention, nicastrin-binding and gamma-secretase activity. EMBO J 23(24):4738–4748. doi:10.1038/sj.emboj.7600478
Kaether C, Scheuermann J, Fassler M, Zilow S, Shirotani K, Valkova C, Novak B, Kacmar S, Steiner H, Haass C (2007) Endoplasmic reticulum retention of the gamma-secretase complex component Pen2 by Rer1. EMBO Rep 8(8):743–748. doi:10.1038/sj.embor.7401027
Spasic D, Raemaekers T, Dillen K, Declerck I, Baert V, Serneels L, Fullekrug J, Annaert W (2007) Rer1p competes with APH-1 for binding to nicastrin and regulates gamma-secretase complex assembly in the early secretory pathway. J Cell Biol 176(5):629–640. doi:10.1083/jcb.200609180
Bonifacino JS, Cosson P, Shah N, Klausner RD (1991) Role of potentially charged transmembrane residues in targeting proteins for retention and degradation within the endoplasmic reticulum. EMBO J 10(10):2783–2793
Berezovska O, Lleo A, Herl LD, Frosch MP, Stern EA, Bacskai BJ, Hyman BT (2005) Familial Alzheimer’s disease presenilin 1 mutations cause alterations in the conformation of presenilin and interactions with amyloid precursor protein. J Neurosci 25(11):3009–3017. doi:10.1523/JNEUROSCI. 0364-05.2005
Chau DM, Crump CJ, Villa JC, Scheinberg DA, Li YM (2012) Familial Alzheimer disease presenilin-1 mutations alter the active site conformation of gamma-secretase. J Biol Chem 287(21):17288–17296. doi:10.1074/jbc.M111.300483
Isoo N, Sato C, Miyashita H, Shinohara M, Takasugi N, Morohashi Y, Tsuji S, Tomita T, Iwatsubo T (2007) Abeta42 overproduction associated with structural changes in the catalytic pore of gamma-secretase: common effects of Pen-2 N-terminal elongation and fenofibrate. J Biol Chem 282(17):12388–12396. doi:10.1074/jbc.M611549200
Querfurth HW, Selkoe DJ (1994) Calcium ionophore increases amyloid beta peptide production by cultured cells. Biochemistry 33(15):4550–4561. doi:10.1021/bi00181a016
Schultz ML, Tecedor L, Chang M, Davidson BL (2011) Clarifying lysosomal storage diseases. Trends Neurosci 34(8):401–410. doi:10.1016/j.tins.2011.05.006
McBrayer M, Nixon RA (2013) Lysosome and calcium dysregulation in Alzheimer’s disease: partners in crime. Biochem Soc Trans 41(6):1495–1502. doi:10.1042/BST20130201
Zhang X, Garbett K, Veeraraghavalu K, Wilburn B, Gilmore R, Mirnics K, Sisodia SS (2012) A role for presenilins in autophagy revisited: normal acidification of lysosomes in cells lacking PSEN1 and PSEN2. J Neurosci 32(25):8633–8648. doi:10.1523/JNEUROSCI. 0556-12.2012
Frykman S, Hur JY, Franberg J, Aoki M, Winblad B, Nahalkova J, Behbahani H, Tjernberg LO (2010) Synaptic and endosomal localization of active gamma-secretase in rat brain. PLoS One 5(1):e8948. doi:10.1371/journal.pone.0008948
Siman R, Flood DG, Thinakaran G, Neumar RW (2001) Endoplasmic reticulum stress-induced cysteine protease activation in cortical neurons: effect of an Alzheimer’s disease-linked presenilin-1 knock-in mutation. J Biol Chem 276(48):44736–44743. doi:10.1074/jbc.M104092200
Liang G, Wang Q, Li Y, Kang B, Eckenhoff MF, Eckenhoff RG, Wei H (2008) A presenilin-1 mutation renders neurons vulnerable to isoflurane toxicity. Anesth Analg 106(2):492–500. doi:10.1213/ane.0b013e3181605b71, table of contents
Janicki SM, Stabler SM, Monteiro MJ (2000) Familial Alzheimer’s disease presenilin-1 mutants potentiate cell cycle arrest. Neurobiol Aging 21(6):829–836. doi:10.1016/S0197-4580(00)00222-0
Janicki SM, Monteiro MJ (1999) Presenilin overexpression arrests cells in the G1 phase of the cell cycle. Arrest potentiated by the Alzheimer’s disease PS2(N141I)mutant. Am J Pathol 155(1):135–144. doi:10.1016/S0002-9440(10)65108-5
Johnston LA, Edgar BA (1998) Wingless and Notch regulate cell-cycle arrest in the developing Drosophila wing. Nature 394(6688):82–84. doi:10.1038/27925
Athan ES, Williamson J, Ciappa A, Santana V, Romas SN, Lee JH, Rondon H, Lantigua RA, Medrano M, Torres M, Arawaka S, Rogaeva E, Song YQ, Sato C, Kawarai T, Fafel KC, Boss MA, Seltzer WK, Stern Y, St George-Hyslop P, Tycko B, Mayeux R (2001) A founder mutation in presenilin 1 causing early-onset Alzheimer disease in unrelated Caribbean Hispanic families. Jama 286(18):2257–2263. doi:10.1001/jama.286.18.2257
Park HK, Na DL, Lee JH, Kim JW, Ki CS (2008) Identification of PSEN1 and APP gene mutations in Korean patients with early-onset Alzheimer’s disease. J Korean Med Sci 23(2):213–217. doi:10.3346/jkms.2008.23.2.213
Goldman JS, Reed B, Gearhart R, Kramer JH, Miller BL (2002) Very early-onset familial Alzheimer’s disease: a novel presenilin 1 mutation. Int J Geriatr Psychiatry 17(7):649–651. doi:10.1002/gps.657
Kim SD, Kim J (2008) Sequence analyses of presenilin mutations linked to familial Alzheimer’s disease. Cell Stress Chaperones 13(4):401–412. doi:10.1007/s12192-008-0046-0
Acknowledgments
We thank Dr. De Strooper for providing PS-null MEF, Dr. Yung-Feng Liao for providing HA-Aph1, Flag-Pen2, wild-type human PS1, and DsRed-GFP-LC3-expressing plasmids, Dr. Liang-Tung Yang for providing NotchΔmyc- and V/LΔmyc-expressing plasmids, and Dr. Rey-Huei Chen for EGFP-Sec61β and EGFP-Rab5 expressing plasmids. This work is supported by Taiwan Ministry of Science and Technology grant (NSC 102-2320-B-010-021-MY2), National Health Research Institute (NHRI-EX98-9816NC), Cheng Hsin General Hospital (102F218C05), Yen Tjing Ling Medical Foundation (CI-102-4), Taipei Veterans General Hospital grant (V103E4-002), and Taiwan Ministry of Education Aim for Top University Grant.
Conflict of Interest
The authors declare that they have no competing interests.
Author Contributions
I.H.C. designed research and analyzed data; W.T.C., Y.F.H., Y.J.H., C.C.L., and Y.T.L. performed research and analyzed data; Y.C.L. and C.C.L. helped with Ca2+ imaging analysis; and W.T.C. and I.H.C. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 3559 kb)
Rights and permissions
About this article
Cite this article
Chen, WT., Hsieh, YF., Huang, YJ. et al. G206D Mutation of Presenilin-1 Reduces Pen2 Interaction, Increases Aβ42/Aβ40 Ratio and Elevates ER Ca2+ Accumulation. Mol Neurobiol 52, 1835–1849 (2015). https://doi.org/10.1007/s12035-014-8969-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8969-1