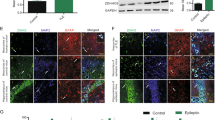
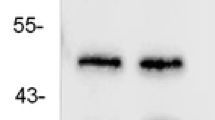
Abstract
Approximately 30 % of epilepsy cases are refractory to current pharmacological treatments. Thus, novel therapeutic approaches that prevent or reverse the molecular and cellular mechanisms of epilepsy are required. 5-HT6 receptor (HTR6) blockade can modulate multiple neurotransmitter systems, and HTR6 may be a potential therapeutic treatment for neurological diseases, including epilepsy. Here, we investigated the role of HTR6 in epilepsy. We detected HTR6 expression both in human epileptic tissues and the pilocarpine rat model by western blotting. We observed behavioral changes after administration of pilocarpine in rats pretreated with a selective HTR6 antagonist, SB-399885, and recorded the electrophysiological index in the pilocarpine rat model pre- or posttreated with SB-399885 by electroencephalogram (EEG) and whole-cell clamp. We measured the activity of mammalian target of rapamycin (mTOR) in the pilocarpine rat model pretreated with the mTOR-specific inhibitor, rapamycin, and SB-399885 using western blotting. We found that HTR6 expression was upregulated in both human tissues and the pilocarpine rat model, and that SB-399885 could suppress epileptic seizures and mTOR activity in epileptic seizures. These results suggest that HTR6 plays an important role in modulating seizure activity and that the blockade of the HTR6/mTOR pathway could be a potential therapeutic target for epilepsy treatment.






Similar content being viewed by others
References
Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR (2011) Incidence of epilepsy: a systematic review and meta-analysis. Neurology 77(10):1005–1012. doi:10.1212/WNL.0b013e31822cfc90
Perucca E, French J, Bialer M (2007) Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol 6(9):793–804. doi:10.1016/S1474-4422(07)70215-6
Stefan H, da Silva FH L, Loscher W, Schmidt D, Perucca E, Brodie MJ, Boon PA, Theodore WH, Moshe SL (2006) Epileptogenesis and rational therapeutic strategies. Acta Neurol Scand 113(3):139–155. doi:10.1111/j.1600-0404.2005.00561.x
Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, Schwartz JC (1993) A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun 193(1):268–276
Yoshioka M, Matsumoto M, Togashi H, Mori K, Saito H (1998) Central distribution and function of 5-HT6 receptor subtype in the rat brain. Life Sci 62(17–18):1473–1477
Marazziti D, Baroni S, Pirone A, Giannaccini G, Betti L, Testa G, Schmid L, Palego L, Borsini F, Bordi F, Piano I, Gargini C, Castagna M, Catena-Dell'osso M, Lucacchini A (2013) Serotonin receptor of type 6 (5-HT6) in human prefrontal cortex and hippocampus post-mortem: an immunohistochemical and immunofluorescence study. Neurochem Int 62(2):182–188. doi:10.1016/j.neuint.2012.11.013
Woolley ML, Marsden CA, Fone KC (2004) 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord 3(1):59–79
Quiedeville A, Boulouard M, Da Silva Costa-Aze V, Dauphin F, Bouet V, Freret T (2014) 5-HT6 receptor antagonists as treatment for age-related cognitive decline. Rev Neurosci. doi:10.1515/revneuro-2014-0013
Routledge C, Bromidge SM, Moss SF, Price GW, Hirst W, Newman H, Riley G, Gager T, Stean T, Upton N, Clarke SE, Brown AM, Middlemiss DN (2000) Characterization of SB-271046: a potent, selective and orally active 5-HT(6) receptor antagonist. Br J Pharmacol 130(7):1606–1612. doi:10.1038/sj.bjp.0703457
Stean TO, Hirst WD, Thomas DR, Price GW, Rogers D, Riley G, Bromidge SM, Serafinowska HT, Smith DR, Bartlett S, Deeks N, Duxon M, Upton N (2002) Pharmacological profile of SB-357134: a potent, selective, brain penetrant, and orally active 5-HT(6) receptor antagonist. Pharmacol Biochem Behav 71(4):645–654
Meffre J, Chaumont-Dubel S, Mannoury La Cour C, Loiseau F, Watson DJ, Dekeyne A, Seveno M, Rivet JM, Gaven F, Deleris P, Herve D, Fone KC, Bockaert J, Millan MJ, Marin P (2012) 5-HT(6) receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol Med 4(10):1043–1056. doi:10.1002/emmm.201201410
Russo E, Citraro R, Donato G, Camastra C, Iuliano R, Cuzzocrea S, Constanti A, De Sarro G, Russo E, Citraro R, Donato G, Camastra C, Iuliano R, Cuzzocrea S, Constanti A, De Sarro G (2013) mTOR inhibition modulates epileptogenesis, seizures and depressive behavior in a genetic rat model of absence epilepsy. Neuropharmacology 69:25–36. doi:10.1016/j.neuropharm.2012.09.019
Wong M (2012) mTOR as a potential treatment target for epilepsy. Future Neurol 7(5):537–545. doi:10.2217/fnl.12.45
Yin H, Wang L, Xiao F, Huang Z, Huang Y, Zhou C, Han Y, Tao S, Yang H, Wang X (2011) Upregulation of liprin-alpha1 protein in the temporal neocortex of intractable epileptic patients and experimental rats. Synapse 65(8):742–750. doi:10.1002/syn.20894
Wang L, Zhou C, Zhu Q, Luo J, Xu Y, Huang Y, Zhang X, Wang X (2010) Up-regulation of serum- and glucocorticoid-induced protein kinase 1 in the brain tissue of human and experimental epilepsy. Neurochem Int 57(8):899–905. doi:10.1016/j.neuint.2010.09.009
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32(3):281–294
Chen G, Kittler JT, Moss SJ, Yan Z (2006) Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J Neurosci 26(9):2513–2521
Zhong P, Gu Z, Wang X, Jiang H, Feng J, Yan Z (2003) Impaired modulation of GABAergic transmission by muscarinic receptors in a mouse transgenic model of Alzheimer's disease. J Biol Chem 278(29):26888–26896
Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, Lan VJ, Joseph A, Jensen FE (2012) The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS One 7(5):e35885. doi:10.1371/journal.pone.0035885 PONE-D-12-02255
Gerard C, Martres MP, Lefevre K, Miquel MC, Verge D, Lanfumey L, Doucet E, Hamon M, el Mestikawy S (1997) Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res 746(1–2):207–219
Riccioni T (2010) 5-HT6 receptor characterization. Int Rev Neurobiol 94:67–88. doi:10.1016/B978-0-12-384976-2.00003-4
Hirst WD, Stean TO, Rogers DC, Sunter D, Pugh P, Moss SF, Bromidge SM, Riley G, Smith DR, Bartlett S, Heidbreder CA, Atkins AR, Lacroix LP, Dawson LA, Foley AG, Regan CM, Upton N (2006) SB-399885 is a potent, selective 5-HT6 receptor antagonist with cognitive enhancing properties in aged rat water maze and novel object recognition models. Eur J Pharmacol 553(1–3):109–119. doi:10.1016/j.ejphar.2006.09.049
Russo E, Citraro R, Constanti A, De Sarro G (2012) The mTOR signaling pathway in the brain: focus on epilepsy and epileptogenesis. Mol Neurobiol 46(3):662–681. doi:10.1007/s12035-012-8314-5
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers are 81201003, 81271445, and 81071040) and funding from the Chongqing Municipal Education Commission (No. KJ 080307). We thank the patients and their families for their participation in this study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors Liang Wang and Yaodong Lv contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Lv, Y., Deng, W. et al. 5-HT6 Receptor Recruitment of mTOR Modulates Seizure Activity in Epilepsy. Mol Neurobiol 51, 1292–1299 (2015). https://doi.org/10.1007/s12035-014-8806-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8806-6