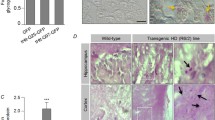
Abstract
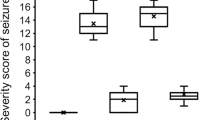
Glycogen, the largest cytosolic macromolecule, is soluble because of intricate construction generating perfect hydrophilic-surfaced spheres. Little is known about neuronal glycogen function and metabolism, though progress is accruing through the neurodegenerative epilepsy Lafora disease (LD) proteins laforin and malin. Neurons in LD exhibit Lafora bodies (LBs), large accumulations of malconstructed insoluble glycogen (polyglucosans). We demonstrated that the laforin–malin complex reduces LBs and protects neuronal cells against endoplasmic reticulum stress-induced apoptosis. We now show that stress induces polyglucosan formation in normal neurons in culture and in the brain. This is mediated by increased glucose-6-phosphate allosterically hyperactivating muscle glycogen synthase (GS1) and is followed by activation of the glycogen digesting enzyme glycogen phosphorylase. In the absence of laforin, stress-induced polyglucosans are undigested and accumulate into massive LBs, and in laforin-deficient mice, stress drastically accelerates LB accumulation and LD. The mechanism through which laforin–malin mediates polyglucosan degradation remains unclear but involves GS1 dephosphorylation by laforin. Our work uncovers the presence of rapid polyglucosan metabolism as part of the normal physiology of neuroprotection. We propose that deficiency in the degradative phase of this metabolism, leading to LB accumulation and resultant seizure predisposition and neurodegeneration, underlies LD.








Similar content being viewed by others
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- CBD:
-
Carbohydrate-binding domain
- 2-DG:
-
2-Deoxyglucose
- ER:
-
Endoplasmic reticulum
- G6P:
-
Glucose-6-phosphate
- GDE1/AGL1:
-
Glycogen debranching enzyme 1
- GSK3:
-
Glycogen synthase kinase 3
- GPBB:
-
Glycogen phosphorylase isoenzyme BB
- GS1:
-
Muscle glycogen synthase
- LB:
-
Lafora body
- LD:
-
Lafora disease
- PB:
-
Polyglucosan body
- N2A:
-
Neuro-2a
- PAS:
-
Periodic acid-Schiff
- PME:
-
Progressive myoclonic epilepsy
- PTG:
-
Protein targeting to glycogen
References
Cavanagh JB (1999) Corpora-amylacea and the family of polyglucosan diseases. Brain Res Brain Res Rev 29:265–295
Gambetti P, Di Mauro S, Hirt L, Blume RP (1971) Myoclonic epilepsy with lafora bodies. Some ultrastructural, histochemical, and biochemical aspects. Arch Neurol 25:483–493
Minassian BA (2002) Progressive myoclonus epilepsy with polyglucosan bodies: Lafora disease. Adv Neurol 89:199–210
Sakai M, Austin J, Witmer F, Trueb L (1970) Studies in myoclonus epilepsy (Lafora body form). II. Polyglucosans in the systemic deposits of myoclonus epilepsy and in corpora amylacea. Neurology 20:160–176
Chan EM, Young EJ, Ianzano L, Munteanu I, Zhao X, Christopoulos CC, Avanzini G, Elia M, Ackerley CA, Jovic NJ, Bohlega S, Andermann E, Rouleau GA, Delgado-Escueta AV, Minassian BA, Scherer SW (2003) Mutations in NHLRC1 cause progressive myoclonus epilepsy. Nat Genet 35:125–127
Minassian BA, Lee JR, Herbrick JA, Huizenga J, Soder S, Mungall AJ, Dunham I, Gardner R, Fong CY, Carpenter S, Jardim L, Satishchandra P, Andermann E, Snead OC 3rd, Lopes-Cendes I, Tsui LC, Delgado-Escueta AV, Rouleau GA, Scherer SW (1998) Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet 20:171–174
Chan EM, Ackerley CA, Lohi H, Ianzano L, Cortez MA, Shannon P, Scherer SW, Minassian BA (2004) Laforin preferentially binds the neurotoxic starch-like polyglucosans, which form in its absence in progressive myoclonus epilepsy. Hum Mol Genet 13:1117–1129
Liu Y, Wang Y, Wu C, Liu Y, Zheng P (2006) Dimerization of Laforin is required for its optimal phosphatase activity, regulation of GSK3beta phosphorylation, and Wnt signaling. J Biol Chem 281:34768–34774
Wang J, Stuckey JA, Wishart MJ, Dixon JE (2002) A unique carbohydrate binding domain targets the lafora disease phosphatase to glycogen. J Biol Chem 277:2377–2380
Worby CA, Gentry MS, Dixon JE (2006) Laforin, a dual specificity phosphatase that dephosphorylates complex carbohydrates. J Biol Chem 281:30412–30418
Tagliabracci VS, Heiss C, Karthik C, Contreras CJ, Glushka J, Ishihara M, Azadi P, Hurley TD, DePaoli-Roach AA, Roach PJ (2011) Phosphate incorporation during glycogen synthesis and Lafora disease. Cell Metab 13:274–282
Tagliabracci VS, Turnbull J, Wang W, Girard JM, Zhao X, Skurat AV, Delgado-Escueta AV, Minassian BA, Depaoli-Roach AA, Roach PJ (2007) Laforin is a glycogen phosphatase, deficiency of which leads to elevated phosphorylation of glycogen in vivo. Proc Natl Acad Sci U S A 104:19262–19266
Liu R, Wang L, Chen C, Liu Y, Zhou P, Wang Y, Wang X, Turnbull J, Minassian BA, Liu Y, Zheng P (2008) Laforin negatively regulates cell cycle progression through glycogen synthase kinase 3beta-dependent mechanisms. Mol Cell Biol 28:7236–7244
Lohi H, Ianzano L, Zhao XC, Chan EM, Turnbull J, Scherer SW, Ackerley CA, Minassian BA (2005) Novel glycogen synthase kinase 3 and ubiquitination pathways in progressive myoclonus epilepsy. Hum Mol Genet 14:2727–2736
Wang Y, Liu Y, Wu C, Zhang H, Zheng X, Zheng Z, Geiger TL, Nuovo GJ, Liu Y, Zheng P (2006) Epm2a suppresses tumor growth in an immunocompromised host by inhibiting Wnt signaling. Cancer Cell 10:179–190
Puri R, Suzuki T, Yamakawa K, Ganesh S (2009) Hyperphosphorylation and aggregation of Tau in laforin-deficient mice, an animal model for Lafora disease. J Biol Chem 284:22657–22663
Gentry MS, Worby CA, Dixon JE (2005) Insights into Lafora disease: malin is an E3 ubiquitin ligase that ubiquitinates and promotes the degradation of laforin. Proc Natl Acad Sci U S A 102:8501–8506
Vilchez D, Ros S, Cifuentes D, Pujadas L, Valles J, Garcia-Fojeda B, Criado-Garcia O, Fernandez-Sanchez E, Medrano-Fernandez I, Dominguez J, Garcia-Rocha M, Soriano E, Rodriguez de Cordoba S, Guinovart JJ (2007) Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci 10:1407–1413
Zeng L, Wang Y, Baba O, Zheng P, Liu Y (2012) Laforin is required for the functional activation of malin in endoplasmic reticulum stress resistance in neuronal cells. FEBS J 279:2467–2478
Brown AM (2004) Brain glycogen re-awakened. J Neurochem 89:537–552
Gruetter R (2003) Glycogen: the forgotten cerebral energy store. J Neurosci Res 74:179–183
Ganesh S, Delgado-Escueta AV, Sakamoto T, Avila MR, Machado-Salas J, Hoshii Y, Akagi T, Gomi H, Suzuki T, Amano K, Agarwala KL, Hasegawa Y, Bai DS, Ishihara T, Hashikawa T, Itohara S, Cornford EM, Niki H, Yamakawa K (2002) Targeted disruption of the Epm2a gene causes formation of Lafora inclusion bodies, neurodegeneration, ataxia, myoclonus epilepsy and impaired behavioral response in mice. Hum Mol Genet 11:1251–1262
Baba O (1993) Production of monoclonal antibody that recognizes glycogen and its application for immunohistochemistry. Kokubyo Gakkai Zasshi 60:264–287
Bian GL, Wei LC, Shi M, Wang YQ, Cao R, Chen LW (2007) Fluoro-Jade C can specifically stain the degenerative neurons in the substantia nigra of the 1-methyl-4-phenyl-1,2,3,6-tetrahydro pyridine-treated C57BL/6 mice. Brain Res 1150:55–61
Schmued LC, Stowers CC, Scallet AC, Xu L (2005) Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 1035:24–31
Worby CA, Gentry MS, Dixon JE (2008) Malin decreases glycogen accumulation by promoting the degradation of protein targeting to glycogen (PTG). J Biol Chem 283:4069–4076
Cervenansky C, Arias A (1984) Glucose-6-phosphate dehydrogenase deficiency in pleiotropic carbohydrate-negative mutant strains of Rhizobium meliloti. J Bacteriol 160:1027–1030
Cabib E, Leloir LF (1958) The biosynthesis of trehalose phosphate. J Biol Chem 231:259–275
Leloir LF, Goldemberg SH (1960) Synthesis of glycogen from uridine diphosphate glucose in liver. J Biol Chem 235:919–923
Spielmann H, Jacob-Muller U, Schulz P (1981) Simple assay of 0.1–1.0 pmol of ATP, ADP, and AMP in single somatic cells using purified luciferin luciferase. Anal Biochem 113:172–178
Maridakis GA, Sotiroudis TG (1996) A catalytic assay for the detection of sub-nanomolar glycogen phosphorylase concentrations. Anal Biochem 240:304–306
Liu Y, Wang Y, Wu C, Zheng P (2009) Deletions and missense mutations of EPM2A exacerbate unfolded protein response and apoptosis of neuronal cells induced by endoplasm reticulum stress. Hum Mol Genet 18:2622–2631
Wang Y, Liu Y, Wu C, McNally B, Liu Y, Zheng P (2008) Laforin confers cancer resistance to energy deprivation-induced apoptosis. Cancer Res 68:4039–4044
Treiman M, Caspersen C, Christensen SB (1998) A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci 19:131–135
Helenius A (1994) How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell 5:253–265
Yu SM, Kim SJ (2010) Endoplasmic reticulum stress (ER-stress) by 2-deoxy-d-glucose (2DG) reduces cyclooxygenase-2 (COX-2) expression and N-glycosylation and induces a loss of COX-2 activity via a Src kinase-dependent pathway in rabbit articular chondrocytes. Exp Mol Med 42:777–786
Zhang K, Kaufman RJ (2006) The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66:S102–S109
DePaoli-Roach AA, Tagliabracci VS, Segvich DM, Meyer CM, Irimia JM, Roach PJ (2010) Genetic depletion of the malin E3 ubiquitin ligase in mice leads to lafora bodies and the accumulation of insoluble laforin. J Biol Chem 285:25372–25381
Tagliabracci VS, Girard JM, Segvich D, Meyer C, Turnbull J, Zhao X, Minassian BA, Depaoli-Roach AA, Roach PJ (2008) Abnormal metabolism of glycogen phosphate as a cause for Lafora disease. J Biol Chem 283:33816–33825
Tiberia E, Turnbull J, Wang T, Ruggieri A, Zhao XC, Pencea N, Israelian J, Wang Y, Ackerley CA, Wang P, Liu Y, Minassian BA (2012) Increased laforin and laforin binding to glycogen underlie lafora body formation in malin-deficient Lafora disease. J Biol Chem 287:25650–25659
Turnbull J, Wang P, Girard JM, Ruggieri A, Wang TJ, Draginov AG, Kameka AP, Pencea N, Zhao X, Ackerley CA, Minassian BA (2010) Glycogen hyperphosphorylation underlies lafora body formation. Ann Neurol 68:925–933
Valles-Ortega J, Duran J, Garcia-Rocha M, Bosch C, Saez I, Pujadas L, Serafin A, Canas X, Soriano E, Delgado-Garcia JM, Gruart A, Guinovart JJ (2011) Neurodegeneration and functional impairments associated with glycogen synthase accumulation in a mouse model of Lafora disease. EMBO Mol Med 3:667–681
Skurat AV, Roach P (2004) Regulation of glycogen synthesis, p. xviii, 1540 p. In: LeRoith D, Taylor SI, Olefsky JM (eds) Diabetes mellitus: a fundamental and clinical text, 3rd edn. Lippincott Williams & Wilkins, Philadelphia
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Inoue M, Yagishita S, Itoh Y, Amano N, Matsushita M (1996) Coexistence of paired helical filaments and polyglucosan bodies in the same neuron in an autopsy case of Alzheimer's disease. Acta Neuropathol 92:511–514
Abubakr A, Wambacq I, Donahue JE, Zappulla R (2005) The presence of polyglucosan bodies in temporal lobe epilepsy: its role and significance. J Clin Neurosci 12:911–914
Acknowledgments
This work was supported by grants from the National Institutes of Health, 1R21NS062391 and 5R21CA164469, and the Canadian Institutes of Health Research to BAM. BAM holds the University of Toronto Michael Bahen chair in epilepsy research.
Conflict of Interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Ma, K., Wang, P. et al. Laforin Prevents Stress-Induced Polyglucosan Body Formation and Lafora Disease Progression in Neurons. Mol Neurobiol 48, 49–61 (2013). https://doi.org/10.1007/s12035-013-8438-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8438-2