Abstract
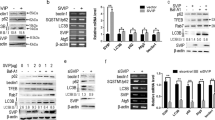
Biliary atresia (BA) is a rare neonatal cholestatic disease that presents with a marked bile duct reaction and rapid fibrotic development. Our earlier research has shown that circUTRN24 is highly elevated in BA, but the exact molecular mechanism is still unknown. This study attempted to investigate whether circUTRN24 induces BA liver fibrosis through regulation of autophagy and to elucidate its molecular mechanism. Using TGF-β-treated hepatic stellate cells (HSC) LX-2, we created a liver fibrosis model. qRT-PCR was used to analyze the expression of circUTRN24, miR-483-3p, and IGF-1. Western blot analysis was used to assess the expression of IGF-1, HSC activation-related proteins, and autophagy-related proteins. The TGF-β-induced LX-2 cell fibrosis model was then supplemented with circUTRN24 siRNA, miR-483-3p mimics, and the autophagy activator Rapamycin, and functional rescue tests were carried out to investigate the role of circUTRN24, miR-483-3p, and autophagy in BA liver fibrosis. Using a luciferase reporter assay, a direct interaction between miR-483-3p and circUTRN24 or IGF-1 was discovered. With the increase of TGF-β treatment concentration, circUTRN24 expression also gradually increased, as did HSC activation and autophagy-related protein. si-circUTRN24 significantly decreased circUTRN24 expression and inhibited HSC activation and autophagy, which was reversed by Rapamycin. Through bioinformatics prediction and validation, we found circUTRN24 might act through miR-483-3p targeting IGF-1 in the autophagy-related mTOR pathway. Furthermore, miR-483-3p mimics significantly increased miR-483-3p expression and inhibited HSC activation and autophagy, which were reversed by Rapamycin. Functional rescue experiments showed that si-circUTRN24 inhibited circUTRN24 and IGF-1 expressions and promoted miR-483-3p expression, while the miR-483-3p inhibitor abolished these effects. These findings imply that circUTRN24/miR-483-3p/IGF-1 axis mediated LX-2 cell fibrosis by regulating autophagy.




Similar content being viewed by others
References
Pal, N., Joy, P. S., & Sergi, C. M. (2022). Biliary atresia animal models: Is the needle in a haystack? International Journal of Molecular Sciences, 23(14), 7838. https://doi.org/10.3390/ijms23147838
Schreiber, R. A., Harpavat, S., Hulscher, J. B. F., & Wildhaber, B. E. (2022). Biliary atresia in 2021: Epidemiology, screening and public policy. Journal of Clinical Medicine, 11(4), 999. https://doi.org/10.3390/jcm11040999
Watanabe, S., Suzuki, T., Tsuchiya, T., & Kondo, Y. (2022). Long-term results of splenomegaly after surgery for biliary atresia in the native liver. Asian Journal of Surgery, 45(3), 849–853. https://doi.org/10.1016/j.asjsur.2021.07.056
Zhu, J. J., Yang, Y. F., Dong, R., & Zheng, S. (2022). Biliatresone: Progress in biliary atresia study. World Journal of Pediatrics. https://doi.org/10.1007/s12519-022-00619-0
Fligor, S. C., Hirsch, T. I., Tsikis, S. T., Adeola, A., & Puder, M. (2022). Current and emerging adjuvant therapies in biliary atresia. Frontiers in Pediatrics, 10, 1007813. https://doi.org/10.3389/fped.2022.1007813
Karpen, S. J., Kelly, D., Mack, C., & Stein, P. (2020). Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders. Hepatology International, 14(5), 677–689. https://doi.org/10.1007/s12072-020-10070-w
Russell, R. C., & Guan, K. L. (2022). The multifaceted role of autophagy in cancer. The EMBO Journal, 41(13), e110031. https://doi.org/10.15252/embj.2021110031
Hou, L. S., Zhang, Y. W., Li, H., Wang, W., Huan, M. L., Zhou, S. Y., & Zhang, B. L. (2022). The regulatory role and mechanism of autophagy in energy metabolism-related hepatic fibrosis. Pharmacology & Therapeutics, 234, 108117. https://doi.org/10.1016/j.pharmthera.2022.108117
Baghaei, K., Mazhari, S., Tokhanbigli, S., Parsamanesh, G., Alavifard, H., Schaafsma, D., & Ghavami, S. (2022). Therapeutic potential of targeting regulatory mechanisms of hepatic stellate cell activation in liver fibrosis. Drug Discovery Today, 27(4), 1044–1061. https://doi.org/10.1016/j.drudis.2021.12.012
Qiu, J. L., Zhang, G. F., Chai, Y. N., Han, X. Y., Zheng, H. T., Li, X. F., Duan, F., & Chen, L. Y. (2022). Ligustrazine attenuates liver fibrosis by targeting miR-145 mediated transforming growth factor-β/smad signaling in an animal model of biliary atresia. Journal of Pharmacology and Experimental Therapeutics, 381(3), 257–265. https://doi.org/10.1124/jpet.121.001020
Hu, Z., Chen, G., Yan, C., Li, Z., Wu, T., Li, L., & Zhang, S. (2023). Autophagy affects hepatic fibrosis progression by regulating macrophage polarization and exosome secretion. Environmental Toxicology. https://doi.org/10.1002/tox.23795
Ma, B., Wang, S., Wu, W., Shan, P., Chen, Y., Meng, J., Xing, L., Yun, J., Hao, L., Wang, X., Li, S., & Guo, Y. (2023). Mechanisms of circRNA/lncRNA-miRNA interactions and applications in disease and drug research. Biomedicine & Pharmacotherapy, 162, 114672. https://doi.org/10.1016/j.biopha.2023.114672
Ruan, H., Wang, P. C., & Han, L. (2023). Characterization of circular RNAs with advanced sequencing technologies in human complex diseases. Wiley Interdisciplinary Reviews: RNA, 14(1), e1759. https://doi.org/10.1002/wrna.1759
Asadi, M. R., Moslehian, M. S., Sabaie, H., Sharifi-Bonab, M., Hakimi, P., Hussen, B. M., Taheri, M., Rakhshan, A., & Rezazadeh, M. (2022). CircRNA-associated CeRNAs regulatory axes in retinoblastoma: A systematic scoping review. Frontiers in Oncology, 12, 910470. https://doi.org/10.3389/fonc.2022.910470
Liu, C. X., & Chen, L. L. (2022). Circular RNAs: Characterization, cellular roles, and applications. Cell, 185(13), 2390. https://doi.org/10.1016/j.cell.2022.06.001
Jiao, S., Wu, S., Huang, S., Liu, M., & Gao, B. (2021). Advances in the identification of circular RNAs and research into circRNAs in human diseases. Frontiers in Genetics, 12, 665233. https://doi.org/10.3389/fgene.2021.665233
Zhang, W., Wu, Z., Chen, S., Zuo, T., Cheng, Z., Fu, J., & Wang, B. (2023). Expression profile of circRNA in biliary atresia and choledochal cyst. Chinese Medical Journal, 136(3), 365–366. https://doi.org/10.1097/cm9.0000000000002079
Wang, T., Liu, D., Gao, J., & Wang, B. (2020). A study on the expression and mechanism of hsa_circ_0009096 and hsa_circ_0083234 in biliary atresia. Chin J Eugen Genet, 28, 1423–1426.
Ye, H. L., Zhang, J. W., Chen, X. Z., Wu, P. B., Chen, L., & Zhang, G. (2020). Ursodeoxycholic acid alleviates experimental liver fibrosis involving inhibition of autophagy. Life Sciences, 242, 117175. https://doi.org/10.1016/j.lfs.2019.117175
Jiang, N., Zhang, J., Ping, J., & Xu, L. (2022). Salvianolic acid B inhibits autophagy and activation of hepatic stellate cells induced by TGF-β1 by downregulating the MAPK pathway. Front Pharmacol, 13, 938856. https://doi.org/10.3389/fphar.2022.938856
Shan, Y., Lu, C., Wang, J., Li, M., Ye, S., Wu, S., Huang, J., Bu, S., & Wang, F. (2022). IGF-1 contributes to liver cancer development in diabetes patients by promoting autophagy. Annals of Hepatology, 27(4), 100697. https://doi.org/10.1016/j.aohep.2022.100697
Wu, W., Wu, W., Ye, Y., Li, T., & Wang, B. (2022). mRNA and lncRNA expression profiles of liver tissues in children with biliary atresia. Experimental and Therapeutic Medicine, 24(4), 634. https://doi.org/10.3892/etm.2022.11571
Ebbesen, K., Hansen, T., & Kjems, J. (2017). Insights into circular RNA biology. RNA Biology, 14(8), 1035–1045. https://doi.org/10.1080/15476286.2016.1271524
Dewidar, B., Meyer, C., Dooley, S., & Meindl-Beinker, A. N. (2019). TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells, 8(11), 1419. https://doi.org/10.3390/cells8111419
Thorne, R. F., Yang, Y., Wu, M., & Chen, S. (2022). TRIMming down autophagy in breast cancer. Autophagy, 18(10), 2512–2513. https://doi.org/10.1080/15548627.2022.2105557
Nakanuma, Y., Sasaki, M., & Harada, K. (2015). Autophagy and senescence in fibrosing cholangiopathies. Journal of Hepatology, 62(4), 934–945. https://doi.org/10.1016/j.jhep.2014.11.027
Xiu, A. Y., Ding, Q., Li, Z., & Zhang, C. Q. (2021). Doxazosin attenuates liver fibrosis by inhibiting autophagy in hepatic stellate cells via activation of the PI3K/Akt/mTOR signaling pathway. Drug Design Development and Therapy, 15, 3643–3659. https://doi.org/10.2147/dddt.s317701
Choi, J., Cho, Y., Choi, H., Lee, S., Han, H., Lee, J., & Kwon, J. (2023). Thymosin beta 4 inhibits LPS and ATP-induced hepatic stellate cells via the regulation of multiple signaling pathways. International Journal of Molecular Sciences, 24(4), 3439. https://doi.org/10.3390/ijms24043439
Nokkeaw, A., Thamjamrassri, P., Tangkijvanich, P., & Ariyachet, C. (2023). Regulatory functions and mechanisms of circular RNAs in hepatic stellate cell activation and liver fibrosis. Cells, 12(3), 378. https://doi.org/10.3390/cells12030378
Liu, Z., Yang, F., Xiao, Z., & Liu, Y. (2023). Review of novel functions and implications of circular RNAs in hepatocellular carcinoma. Frontiers in Oncology, 13, 1093063. https://doi.org/10.3389/fonc.2023.1093063
Yan, J., Ye, G., Jin, Y., Miao, M., Li, Q., & Zhou, H. (2023). Identification of novel prognostic circRNA biomarkers in circRNA-miRNA-mRNA regulatory network in gastric cancer and immune infiltration analysis. BMC Genomics, 24(1), 323. https://doi.org/10.1186/s12864-023-09421-2
Ding, Y., Chen, Y., Hu, K., Yang, Q., Li, Y., & Huang, M. (2023). Sweroside alleviates hepatic steatosis in part by activating AMPK/mTOR-mediated autophagy in mice. Journal of Cellular Biochemistry. https://doi.org/10.1002/jcb.30428
Sun, M., Gong, P., Yuan, B., Liu, N., Li, X., Zhang, W., & Wang, M. (2023). AXL-Induced Autophagy mitigates experimental autoimmune encephalomyelitis by suppressing microglial inflammation via the PI3K/AKT/mTOR signaling pathway. Molecular Immunology, 159, 15–27. https://doi.org/10.1016/j.molimm.2023.05.005
Tan, Y., Li, C., Zhou, J., Deng, F., & Liu, Y. (2023). Berberine attenuates liver fibrosis by autophagy inhibition triggering apoptosis via the miR-30a-5p/ATG5 axis. Experimental Cell Research, 427(2), 113600. https://doi.org/10.1016/j.yexcr.2023.113600
Cheng, R., Xu, H., & Hong, Y. (2021). miR221 regulates TGF-β1-induced HSC activation through inhibiting autophagy by directly targeting LAMP2. Molecular Medicine Reports, 24(5), 777. https://doi.org/10.3892/mmr.2021.12417
Luo, G., Wang, X., & Liu, C. (2022). MiR-483–3p improves learning and memory abilities via XPO1 in Alzheimer’s disease. Brain and Behavior, 12(8), e2680. https://doi.org/10.1002/brb3.2680
Yuan, L., Zhang, P., Lu, Y., Zhang, A., & Chen, X. (2022). LINC00662 promotes proliferation and invasion and inhibits apoptosis of glioma cells through miR-483-3p/SOX3 axis. Applied Biochemistry and Biotechnology, 194(7), 2857–2871. https://doi.org/10.1007/s12010-022-03855-2
Shang, F., Guo, X., Chen, Y., Wang, C., Gao, J., Wen, E., Lai, B., & Bai, L. (2022). Endothelial microRNA-483-3p is hypertension-protective. Oxidative Medicine and Cellular Longevity, 2022, 3698219. https://doi.org/10.1155/2022/3698219
Bu, X., Ding, W., Guo, S., Wang, Y., Feng, J., Wang, P., Chen, Y., & Ge, Z. (2023). Differential expression of microRNAs in bile duct obstruction-induced liver fibrosis and the identification of a novel liver fibrosis marker miR-1295b-3p. Annals of Translational Medicine, 11(1), 22. https://doi.org/10.21037/atm-22-6416
Li, F., Ma, N., Zhao, R., Wu, G., Zhang, Y., Qiao, Y., Han, D., Xu, Y., Xiang, Y., Yan, B., Jin, J., Lv, G., Wang, L., Xu, C., Gao, X., & Luo, S. (2014). Overexpression of miR-483-5p/3p cooperate to inhibit mouse liver fibrosis by suppressing the TGF-β stimulated HSCs in transgenic mice. Journal of Cellular and Molecular Medicine, 18(6), 966–974. https://doi.org/10.1111/jcmm.12293
Dabin, R., Wei, C., Liang, S., Ke, C., Zhihan, W., & Ping, Z. (2022). Astrocytic IGF-1 and IGF-1R orchestrate mitophagy in traumatic brain injury via exosomal miR-let-7e. Oxidative Medicine and Cellular Longevity, 2022, 3504279. https://doi.org/10.1155/2022/3504279
Zhou, J., Lin, J., Zhao, Y., & Sun, X. (2022). Deregulated expression of miR-483-3p serves as a diagnostic biomarker in severe pneumonia children with respiratory failure and its predictive value for the clinical outcome of patients. Molecular Biotechnology, 64(3), 311–319. https://doi.org/10.1007/s12033-021-00415-7
Han, L., Luo, Q. Q., Peng, M. G., Zhang, Y., & Zhu, X. H. (2021). miR-483 is downregulated in pre-eclampsia via targeting insulin-like growth factor 1 (IGF1) and regulates the PI3K/Akt/mTOR pathway of endothelial progenitor cells. The Journal of Obstetrics and Gynaecology Research, 47(1), 63–72. https://doi.org/10.1111/jog.14412
Li, Y., Tian, Z., Pan, G., Zhao, P., Pan, D., Zhang, J., Ye, L., Zhang, F., & Xu, X. (2022). Heidihuangwan alleviates renal fibrosis in rats with 5/6 nephrectomy by inhibiting autophagy. Frontiers in pharmacology, 13, 977284. https://doi.org/10.3389/fphar.2022.977284
Shi, H., Shi, H., Ren, F., Chen, D., Chen, Y., & Duan, Z. (2017). Naringin in Ganshuang Granule suppresses activation of hepatic stellate cells for anti-fibrosis effect by inhibition of mammalian target of rapamycin. Journal of cellular and molecular medicine, 21(3), 500–509. https://doi.org/10.1111/jcmm.12994
Funding
This project supported by the Special Research Foundation of Shenzhen Children’s Hospital of China (ynkt2020-zz12) and Medical Scientific Research Foundation of Guangdong Province of China (A2021364).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, D., Wu, Z., Gao, J. et al. CircUTRN24/miR-483-3p/IGF-1 Regulates Autophagy Mediated Liver Fibrosis in Biliary Atresia. Mol Biotechnol 66, 1424–1433 (2024). https://doi.org/10.1007/s12033-023-00802-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00802-2