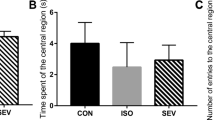
Abstract
An increasing number of studies reveal the deleterious effects of isoflurane (Iso) exposure during pregnancy on offspring cognition. However, no effective therapeutic strategy for Iso-induced deleterious effects has been well developed. Angelicin exerts an anti-inflammatory effect on neurons and glial cells. This study investigated the roles and mechanism of action of angelicin in Iso-induced neurotoxicity in vitro and in vivo. After exposing C57BL/6 J mice on embryonic day 15 (E15) to Iso for 3 and 6 h, respectively, neonatal mice on embryonic day 18 (E18) displayed obvious anesthetic neurotoxicity, which was revealed by the elevation of cerebral inflammatory factors and blood–brain barrier (BBB) permeability and cognitive dysfunction in mice. Angelicin treatment could not only significantly reduce the Iso-induced embryonic inflammation and BBB disruption but also improve the cognitive dysfunction of offspring mice. Iso exposure resulted in an increase of carbonic anhydrase (CA) 4 and aquaporin-4 (AQP4) expression at both mRNA and protein levels in vascular endothelial cells and mouse brain tissue collected from neonatal mice on E18. Remarkably, the Iso-induced upregulation of CA4 and AQP4 expression could be partially reversed by angelicin treatment. Moreover, GSK1016790A, an AQP4 agonist, was used to confirm the role of AQP4 in the protective effect of angelicin. Results showed that GSK1016790A abolished the therapeutic effect of angelicin on Iso-induced inflammation and BBB disruption in the embryonic brain and on the cognitive function of offspring mice. In conclusion, angelicin may serve as a potential therapeutic for Iso-induced neurotoxicity in neonatal mice by regulating the CA4/AQP4 pathway.
Graphical Abstract






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- Iso:
-
Isoflurane
- E15:
-
Embryonic day 15
- E18:
-
Embryonic day 18
- BBB:
-
Blood–brain barrier
- CA:
-
Carbonic anhydrase
- AQP4:
-
Aquaporin-4
- P7:
-
Postnatal day 7
- P21:
-
Postnatal day 21
References
Kang, E., Wen, Z., Song, H., Christian, K. M., & Ming, G. L. (2016). Adult neurogenesis and psychiatric disorders. Cold Spring Harbor Perspectives Biology, 8, a019026.
Jevtovic-Todorovic, V., Hartman, R. E., Izumi, Y., Benshoff, N. D., Dikranian, K., Zorumski, C. F., Olney, J. W., & Wozniak, D. F. (2003). Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. Journal of Neuroscience, 23, 876–882.
Cheng, Y., He, L., Prasad, V., Wang, S., & Levy, R. J. (2015). Anesthesia-induced neuronal apoptosis in the developing retina: A window of opportunity. Anesthesia and Analgesia, 121, 1325–1335.
Dittmar, M. S., Petermichl, W., Schlachetzki, F., Graf, B. M., & Gruber, M. (2012). Isoflurane induces endothelial apoptosis of the post-hypoxic blood-brain barrier in a transdifferentiated human umbilical vein endothelial cell model. PLoS ONE, 7, e38260.
Acharya, N. K., Goldwaser, E. L., Forsberg, M. M., Godsey, G. A., Johnson, C. A., Sarkar, A., DeMarshall, C., Kosciuk, M. C., Dash, J. M., Hale, C. P., Leonard, D. M., Appelt, D. M., & Nagele, R. G. (2015). Sevoflurane and Isoflurane induce structural changes in brain vascular endothelial cells and increase blood-brain barrier permeability: Possible link to postoperative delirium and cognitive decline. Brain Research, 1620, 29–41.
He, H. J., Wang, Y., Le, Y., Duan, K. M., Yan, X. B., Liao, Q., Liao, Y., Tong, J. B., Terrando, N., & Ouyang, W. (2012). Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neuroscience & Therapeutics, 18, 994–1002.
Shin Low, S., Nong Lim, C., Yew, M., Siong Chai, W., Low, L. E., Manickam, S., Ti Tey, B., & Show, P. L. (2021). Recent ultrasound advancements for the manipulation of nanobiomaterials and nanoformulations for drug delivery. Ultrasonics Sonochemistry, 80, 105805.
Huo, S., Liao, Z., Zhao, P., Zhou, Y., Göstl, R., & Herrmann, A. (2022). Mechano-Nanoswitches for Ultrasound-Controlled Drug Activation. Advanced Science, 9, e2104696.
Tiberi, S., du Plessis, N., Walzl, G., Vjecha, M. J., Rao, M., Ntoumi, F., Mfinanga, S., Kapata, N., Mwaba, P., McHugh, T. D., Ippolito, G., Migliori, G. B., Maeurer, M. J., & Zumla, A. (2018). Tuberculosis: Progress and advances in development of new drugs, treatment regimens, and host-directed therapies. The Lancet Infectious Diseases, 18, e183–e198.
Mahendra, C. K., Tan, L. T. H., Lee, W. L., Yap, W. H., Pusparajah, P., Low, L. E., Tang, S. Y., Chan, K. G., Lee, L. H., & Goh, B. H. (2020). Angelicin-A furocoumarin compound with vast biological potential. Frontiers in Pharmacology, 11, 366.
Wei, D. Z., Guo, X. Y., Lin, L. N., Lin, M. X., Gong, Y. Q., Ying, B. Y., & Huang, M. Y. (2016). Effects of angelicin on ovalbumin (OVA)-induced airway inflammation in a mouse model of asthma. Inflammation, 39, 1876–1882.
Liu, F., Sun, G. Q., Gao, H. Y., Li, R. S., Soromou, L. W., Chen, N., Deng, Y. H., & Feng, H. H. (2013). Angelicin regulates LPS-induced inflammation via inhibiting MAPK/NF-kappaB pathways. Journal of Surgical Research, 185, 300–309.
Nirmaladevi, D., Venkataramana, M., Chandranayaka, S., Ramesha, A., Jameel, N. M., & Srinivas, C. (2014). Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cellular and Molecular Neurobiology, 34, 973–985.
Okuyama, T., Batanian, J. R., & Sly, W. S. (1993). Genomic organization and localization of gene for human carbonic anhydrase IV to chromosome 17q. Genomics, 16, 678–684.
Ghandour, M. S., Langley, O. K., Zhu, X. L., Waheed, A., & Sly, W. S. (1992). Carbonic anhydrase IV on brain capillary endothelial cells: A marker associated with the blood-brain barrier. Proceedings of the National Academy of Sciences USA, 89, 6823–6827.
Lemon, N., Canepa, E., Ilies, M. A., & Fossati, S. (2021). Carbonic anhydrases as potential targets against neurovascular unit dysfunction in Alzheimer’s disease and stroke. Front Aging Neurosci, 13, 772278.
Salameh, T. S., Mortell, W. G., Logsdon, A. F., Butterfield, D. A., & Banks, W. A. (2019). Disruption of the hippocampal and hypothalamic blood-brain barrier in a diet-induced obese model of type II diabetes: Prevention and treatment by the mitochondrial carbonic anhydrase inhibitor, topiramate. Fluids Barriers CNS, 16, 1.
Nicchia, G. P., Nico, B., Camassa, L. M., Mola, M. G., Loh, N., Dermietzel, R., Spray, D. C., Svelto, M., & Frigeri, A. (2004). The role of aquaporin-4 in the blood-brain barrier development and integrity: Studies in animal and cell culture models. Neuroscience, 129, 935–945.
Nagelhus, E. A., Mathiisen, T. M., & Ottersen, O. P. (2004). Aquaporin-4 in the central nervous system: Cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience, 129, 905–913.
Vilas, G., Krishnan, D., Loganathan, S. K., Malhotra, D., Liu, L., Beggs, M. R., Gena, P., Calamita, G., Jung, M., Zimmermann, R., Tamma, G., Casey, J. R., & Alexander, R. T. (2015). Increased water flux induced by an aquaporin-1/carbonic anhydrase II interaction. Molecular Biology of the Cell, 26, 1106–1118.
Demirgan, S., Akyol, O., Temel, Z., Sengelen, A., Pekmez, M., Demirgan, R., Sevdi, M. S., Erkalp, K., & Selcan, A. (2019). Isoflurane exposure in infant rats acutely increases aquaporin 4 and does not cause neurocognitive impairment. Bosnian Journal of Basic Medical Sciences, 19, 257–264.
Hirabayashi, Y., & Gotoh, Y. (2005). Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neuroscience Research, 51, 331–336.
Xu, S., Liu, B., Yin, M., Koroleva, M., Mastrangelo, M., Ture, S., Morrell, C. N., Zhang, D. X., Fisher, E. A., & Jin, Z. G. (2016). A novel TRPV4-specific agonist inhibits monocyte adhesion and atherosclerosis. Oncotarget, 7, 37622–37635.
Vorhees, C. V., & Williams, M. T. (2006). Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nature Protocols, 1, 848–858.
Seibenhener, M. L., & Wooten, M. C. (2015). Use of the open field maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments, 96, e52434.
Salman, M. M., Kitchen, P., Woodroofe, M. N., Brown, J. E., Bill, R. M., Conner, A. C., & Conner, M. T. (2017). Hypothermia increases aquaporin 4 (AQP4) plasma membrane abundance in human primary cortical astrocytes via a calcium/transient receptor potential vanilloid 4 (TRPV4)- and calmodulin-mediated mechanism. European Journal of Neuroscience, 46, 2542–2547.
Li, X., Jiang, X., & Zhao, P. (2020). Effects of pregnancy anesthesia on fetal nervous system. Frontiers in Pharmacology, 11, 523514.
Wu, Z., Li, X., Zhang, Y., Tong, D., Wang, L., & Zhao, P. (2018). Effects of sevoflurane exposure during mid-pregnancy on learning and memory in offspring rats: Beneficial effects of maternal exercise. Frontiers in Cellular Neuroscience, 12, 122.
Zuo, Y., Li, B., Xie, J., Ma, Z., Thirupathi, A., Yu, P., Gao, G., Zhou, J., Zhou, C., Xu, H., Chang, Y., & Shi, Z. (2020). Sevoflurane anesthesia during pregnancy in mice induces cognitive impairment in the offspring by causing iron deficiency and inhibiting myelinogenesis. Neurochemistry International, 135, 104693.
Huang, W., Dong, Y., Zhao, G., Wang, Y., Jiang, J., & Zhao, P. (2018). Influence of isoflurane exposure in pregnant rats on the learning and memory of offsprings. BMC Anesthesiology, 18, 5.
Kong, F. J., Ma, L. L., Hu, W. W., Wang, W. N., Lu, H. S., & Chen, S. P. (2012). Fetal exposure to high isoflurane concentration induces postnatal memory and learning deficits in rats. Biochemical Pharmacology, 84, 558–563.
Li, Y., Liang, G., Wang, S., Meng, Q., Wang, Q., & Wei, H. (2007). Effects of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology, 53, 942–950.
Palanisamy, A., Baxter, M. G., Keel, P. K., Xie, Z., Crosby, G., & Culley, D. J. (2011). Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology, 114, 521–528.
Sanders, R. D., Xu, J., Shu, Y., Januszewski, A., Halder, S., Fidalgo, A., Sun, P., Hossain, M., Ma, D., & Maze, M. (2009). Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology, 110, 1077–1085.
Chen, Y., Lian, F., Lu, Q., Peng, S., Li, J., Huang, S., & Du, X. (2020). L-Theanine attenuates isoflurane-induced injury in neural stem cells and cognitive impairment in neonatal mice. Biological & Pharmaceutical Bulletin, 43, 938–945.
Uchida, Y., Hashimoto, T., Saito, H., Takita, K., & Morimoto, Y. (2022). Neonatal isoflurane exposure disturbs granule cell migration in the rat dentate gyrus. Biomedical research (Tokyo, Japan), 43, 1–9.
Broad, K. D., Hassell, J., Fleiss, B., Kawano, G., Ezzati, M., Rocha-Ferreira, E., Hristova, M., Bennett, K., Fierens, I., Burnett, R., Chaban, B., Alonso-Alconada, D., Oliver-Taylor, A., Tachsidis, I., Rostami, J., Gressens, P., Sanders, R. D., & Robertson, N. J. (2016). Isoflurane exposure induces cell death, microglial activation and modifies the expression of genes supporting neurodevelopment and cognitive function in the male newborn piglet Brain. PLoS ONE, 11, e0166784.
Wu, L., Zhao, H., Weng, H., & Ma, D. (2019). Lasting effects of general anesthetics on the brain in the young and elderly: “mixed picture” of neurotoxicity, neuroprotection and cognitive impairment. Journal of Anesthesia, 33, 321–335.
Zhang, Y., Wu, Z., Li, X., Wan, Y., Zhang, Y., & Zhao, P. (2020). Maternal sevoflurane exposure affects differentiation of hippocampal neural stem cells by regulating miR-410-3p and ATN1. Stem Cell Research & Therapy, 11, 423.
Wen, J., Xu, J., Mathena, R. P., Choi, J. H., & Mintz, C. D. (2021). Early isoflurane exposure impairs synaptic development in Fmr1 KO mice via the mTOR pathway. Neurochemical Research, 46, 1577–1588.
Liu, H., Dai, T., & Guo, W. (2013). Isoflurane-induced neuronal apoptosis in developing hippocampal neurons. Neural Regeneration Research, 8, 825–832.
Hudalla, H., Michael, Z., Christodoulou, N., Willis, G. R., Fernandez-Gonzalez, A., Filatava, E. J., Dieffenbach, P., Fredenburgh, L. E., Stearman, R. S., Geraci, M. W., Kourembanas, S., & Christou, H. (2019). Carbonic anhydrase inhibition ameliorates inflammation and experimental pulmonary hypertension. American Journal of Respiratory Cell and Molecular Biology, 61, 512–524.
Provensi, G., Nocentini, A., Passani, M. B., Blandina, P., & Supuran, C. T. (2021). Activation of carbonic anhydrase isoforms involved in modulation of emotional memory and cognitive disorders with histamine agonists, antagonists and derivatives. Journal of Enzyme Inhibition and Medicinal Chemistry, 36, 719–726.
Puscas, I., Reznicek, A., Moldovan, A., Puscas, C., & Sturzu, L. (1985). Activation of carbonic anhydrase by beta-adrenergic agonists and inhibition by beta-adrenergic blockers. Médecine Interne, 23, 185–189.
Lunardi, N., Hucklenbruch, C., Latham, J. R., Scarpa, J., & Jevtovic-Todorovic, V. (2011). Isoflurane impairs immature astroglia development in vitro: The role of actin cytoskeleton. Journal of Neuropathology and Experimental Neurology, 70, 281–291.
Culley, D. J., Cotran, E. K., Karlsson, E., Palanisamy, A., Boyd, J. D., Xie, Z., & Crosby, G. (2013). Isoflurane affects the cytoskeleton but not survival, proliferation, or synaptogenic properties of rat astrocytes in vitro. British Journal of Anaesthesia, 110(Suppl 1), i19-28.
Acknowledgements
None.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Miao, M. & Wei, F. Angelicin Alleviates Maternal Isoflurane Exposure-Induced Offspring Cognitive Defects Through the Carbonic Anhydrase 4/Aquaporin-4 Pathway. Mol Biotechnol 66, 34–43 (2024). https://doi.org/10.1007/s12033-023-00723-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-00723-0