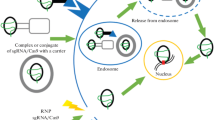
Abstract
The efficient delivery of CRISPR-Cas components is still a key and unsolved problem. CRISPR-Cas delivery in the form of a Cas protein+sgRNA (ribonucleoprotein complex, RNP complex), has proven to be extremely effective, since it allows to increase on-target activity, while reducing nonspecific activity. The key point for in vivo genome editing is the direct delivery of artificial nucleases and donor DNA molecules into the somatic cells of an adult organism. At the same time, control of the dose of artificial nucleases is impossible, which affects the efficiency of genome editing in the affected cells. Poor delivery efficiency and low editing efficacy reduce the overall potency of the in vivo genome editing process. Here we review how this problem is currently being solved in scientific works and what types of in vivo delivery methods of Cas9/sgRNA RNPs have been developed.

Similar content being viewed by others
References
Ishino, Y., Shinagawa, H., Makino, K., Amemura, M., & Nakatura, A. (1987). Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isoenzyme conversion in Escherichia coli, and identification of the gene product. Journal of Bacteriology, 169(12), 5429–5433. https://doi.org/10.1128/jb.169.12.5429-5433.1987
Jansen, R., Van Embden, J. D. A., Gaastra, W., & Schouls, L. M. (2002). Identification of genes that are associated with DNA repeats in prokaryotes. Molecular Microbiology, 43(6), 1565–1575. https://doi.org/10.1046/j.1365-2958.2002.02839.x
Mojica, F. J. M., Díez-Villaseñor, C., García-Martínez, J., & Soria, E. (2005). Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. Journal of Molecular Evolution, 60(2), 174–182. https://doi.org/10.1007/s00239-004-0046-3
McGinn, J., & Marraffini, L. A. (2019). Molecular mechanisms of CRISPR–Cas spacer acquisition. Nature Reviews Microbiology. https://doi.org/10.1038/s41579-018-0071-7
Gasiunas, G., Barrangou, R., Horvath, P., & Siksnys, V. (2012). Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.1208507109
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 337(6096), 816–821. https://doi.org/10.1126/science.1225829
Heler, R., Samai, P., Modell, J. W., Weiner, C., Goldberg, G. W., Bikard, D., & Marraffini, L. A. (2015). Cas9 specifies functional viral targets during CRISPR-Cas adaptation. Nature, 519(7542), 199–202. https://doi.org/10.1038/nature14245
Barrangou, R., & Doudna, J. A. (2016). Applications of CRISPR technologies in research and beyond. Nature Biotechnology, 34(9), 933–941. https://doi.org/10.1038/nbt.3659
Komor, A. C., Badran, A. H., & Liu, D. R. (2017). CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. https://doi.org/10.1016/j.cell.2016.10.044
Gao, X., Tao, Y., Lamas, V., Huang, M., Yeh, W. H., Pan, B., Hu, Y. J., Hu, J. H., Thompson, D. B., Shu, Y., Li, Y., Wang, H., Yang, S., Xu, Q., Polley, D. B., Liberman, M. C., Kong, W. J., Holt, J. R., Chen, Z. Y., & Liu, D. R. (2018). Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature, 553(7687), 217–221. https://doi.org/10.1038/nature25164
Park, C. Y., Kim, D. H., Son, J. S., Sung, J. J., Lee, J., Bae, S., Kim, J. H., Kim, D. W., & Kim, J. S. (2015). Functional correction of large factor VIII gene chromosomal inversions in hemophilia a patient-derived iPSCs using CRISPR-Cas9. Cell Stem Cell, 17(2), 213–220. https://doi.org/10.1016/j.stem.2015.07.001
Liang, P., Xu, Y., Zhang, X., Ding, C., Huang, R., Zhang, Z., Lv, J., Xie, X., Chen, Y., Li, Y., Sun, Y., Bai, Y., Songyang, Z., Ma, W., Zhou, C., & Huang, J. (2015). CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein and Cell, 6(5), 363–372. https://doi.org/10.1007/s13238-015-0153-5
Xie, F., Ye, L., Chang, J. C., Beyer, A. I., Wang, J., Muench, M. O., & Kan, Y. W. (2014). Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Research, 24(9), 1526–1533. https://doi.org/10.1101/gr.173427.114
Yin, H., Xue, W., Chen, S., Bogorad, R. L., Benedetti, E., Grompe, M., Koteliansky, V., Sharp, P. A., Jacks, T., & Anderson, D. G. (2014). Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nature Biotechnology, 32(6), 551–553. https://doi.org/10.1038/nbt.2884
Genovese, P., Schiroli, G., Escobar, G., Di Tomaso, T., Firrito, C., Calabria, A., Moi, D., Mazzieri, R., Bonini, C., Holmes, M. C., Gregory, P. D., Van Der Burg, M., Gentner, B., Montini, E., Lombardo, A., & Naldini, L. (2014). Targeted genome editing in human repopulating haematopoietic stem cells. Nature, 510(7504), 235–240. https://doi.org/10.1038/nature13420
Liu, R., Paxton, W. A., Choe, S., Ceradini, D., Martin, S. R., Horuk, R., MacDonald, M. E., Stuhlmann, H., Koup, R. A., & Landau, N. R. (1996). Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell, 86(3), 367–377. https://doi.org/10.1016/S0092-8674(00)80110-5
Long, C., Amoasii, L., Mireault, A. A., McAnally, J. R., Li, H., Sanchez-Ortiz, E., Bhattacharyya, S., Shelton, J. M., Bassel-Duby, R., & Olson, E. N. (2016). Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science, 351(6271), 400–403. https://doi.org/10.1126/science.aad5725
Nelson, C. E., Hakim, C. H., Ousterout, D. G., Thakore, P. I., Moreb, E. A., Castellanos Rivera, R. M., Madhavan, S., Pan, X., Ran, F. A., Yan, W. X., Asokan, A., Zhang, F., Duan, D., & Gersbach, C. A. (2016). In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science, 351(6271), 403–407. https://doi.org/10.1126/science.aad5143
Tabebordbar, M., Zhu, K., Cheng, J. K. W., Chew, W. L., Widrick, J. J., Yan, W. X., Maesner, C., Wu, E. Y., Xiao, R., Ran, F. A., Cong, L., Zhang, F., Vandenberghe, L. H., Church, G. M., & Wagers, A. J. (2016). In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science, 351(6271), 407–411. https://doi.org/10.1126/science.aad5177
Monteys, A. M., Ebanks, S. A., Keiser, M. S., & Davidson, B. L. (2017). CRISPR/cas9 editing of the mutant huntingtin allele in vitro and in vivo. Molecular Therapy, 25(1), 12–23. https://doi.org/10.1016/j.ymthe.2016.11.010
Cox, D. B. T., Platt, R. J., & Zhang, F. (2015). Therapeutic genome editing: Prospects and challenges. Nature Medicine. https://doi.org/10.1038/nm.3793
Ghaemi, A., Bagheri, E., Abnous, K., Taghdisi, S. M., Ramezani, M., & Alibolandi, M. (2021). CRISPR-cas9 genome editing delivery systems for targeted cancer therapy. Life Sciences, 267, 118969. https://doi.org/10.1016/J.LFS.2020.118969
Martella, A., Firth, M., Taylor, B. J. M., Göppert, A., Cuomo, E. M., Roth, R. G., Dickson, A. J., & Fisher, D. I. (2019). Systematic evaluation of CRISPRa and CRISPRi modalities enables development of a multiplexed, orthogonal gene activation and repression system. ACS Synthetic Biology, 8(9), 1998–2006. https://doi.org/10.1021/acssynbio.8b00527
Kampmann, M. (2018). CRISPRi and CRISPRa screens in mammalian cells for precision biology and medicine. ACS Chemical Biology, 13(2), 406–416. https://doi.org/10.1021/acschembio.7b00657
Li, C., Brant, E., Budak, H., & Zhang, B. (2021). CRISPR/Cas: A Nobel Prize award-winning precise genome editing technology for gene therapy and crop improvement. Journal of Zhejiang University-Science B, 22(4), 253–284. https://doi.org/10.1631/JZUS.B2100009
Shalaby, K., Aouida, M., & El-Agnaf, O. (2020). Tissue-specific delivery of crispr therapeutics: Strategies and mechanisms of non-viral vectors. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms21197353
Mendell, J. R., Al-Zaidy, S. A., Rodino-Klapac, L. R., Goodspeed, K., Gray, S. J., Kay, C. N., Boye, S. L., Boye, S. E., George, L. A., Salabarria, S., Corti, M., Byrne, B. J., & Tremblay, J. P. (2021). Current clinical applications of in vivo gene therapy with AAVs. Molecular Therapy. https://doi.org/10.1016/j.ymthe.2020.12.007
Chuang, Y. F., Phipps, A. J., Lin, F. L., Hecht, V., Hewitt, A. W., Wang, P. Y., & Liu, G. S. (2021). Approach for in vivo delivery of CRISPR/Cas system: A recent update and future prospect. Cellular and Molecular Life Sciences. https://doi.org/10.1007/s00018-020-03725-2
Ashmore-Harris, C., & Fruhwirth, G. O. (2020). The clinical potential of gene editing as a tool to engineer cell-based therapeutics. Clinical and Translational Medicine. https://doi.org/10.1186/s40169-020-0268-z
Kunz, J. B., & Kulozik, A. E. (2020). Gene therapy of the hemoglobinopathies. HemaSphere, 4(5), e479. https://doi.org/10.1097/hs9.0000000000000479
Luthra, R., Kaur, S., & Bhandari, K. (2021). Applications of CRISPR as a potential therapeutic. Life Sciences. https://doi.org/10.1016/J.LFS.2021.119908
Kay, M. A. (2011). State-of-the-art gene-based therapies: The road ahead. Nature Reviews Genetics. https://doi.org/10.1038/nrg2971
Li, H., Haurigot, V., Doyon, Y., Li, T., Wong, S. Y., Bhagwat, A. S., Malani, N., Anguela, X. M., Sharma, R., Ivanciu, L., Murphy, S. L., Finn, J. D., Khazi, F. R., Zhou, S., Paschon, D. E., Rebar, E. J., Bushman, F. D., Gregory, P. D., Holmes, M. C., & High, K. A. (2011). In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature, 475(7355), 217–221. https://doi.org/10.1038/nature10177
Yin, H., Song, C. Q., Dorkin, J. R., Zhu, L. J., Li, Y., Wu, Q., Park, A., Yang, J., Suresh, S., Bizhanova, A., Gupta, A., Bolukbasi, M. F., Walsh, S., Bogorad, R. L., Gao, G., Weng, Z., Dong, Y., Koteliansky, V., Wolfe, S. A., … Anderson, D. G. (2016). Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nature Biotechnology, 34(3), 328–333. https://doi.org/10.1038/nbt.3471
Cohen, J., Pertsemlidis, A., Kotowski, I. K., Graham, R., Garcia, C. K., & Hobbs, H. H. (2005). Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nature Genetics, 37(2), 161–165. https://doi.org/10.1038/ng1509
Ran, F. A., Hsu, P. D., Lin, C. Y., Gootenberg, J. S., Konermann, S., Trevino, A. E., Scott, D. A., Inoue, A., Matoba, S., Zhang, Y., & Zhang, F. (2013). Double nicking by RNA-guided CRISPR cas9 for enhanced genome editing specificity. Cell, 154(6), 1380–1389. https://doi.org/10.1016/j.cell.2013.08.021
Ding, Q., Strong, A., Patel, K. M., Ng, S. L., Gosis, B. S., Regan, S. N., Cowan, C. A., Rader, D. J., & Musunuru, K. (2014). Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circulation Research, 115(5), 488–492. https://doi.org/10.1161/CIRCRESAHA.115.304351
Kim, S., Kim, D., Cho, S. W., Kim, J., & Kim, J. S. (2014). Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Research, 24(6), 1012–1019. https://doi.org/10.1101/gr.171322.113
Lin, S., Staahl, B. T., Alla, R. K., & Doudna, J. A. (2014). Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife, 3, e04766. https://doi.org/10.7554/eLife.04766
Fu, Y., Sander, J. D., Reyon, D., Cascio, V. M., & Joung, J. K. (2014). Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nature Biotechnology, 32(3), 279–284. https://doi.org/10.1038/nbt.2808
Cho, S. W., Kim, S., Kim, Y., Kweon, J., Kim, H. S., Bae, S., & Kim, J. S. (2014). Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Research, 24(1), 132–141. https://doi.org/10.1101/gr.162339.113
Liang, X., Potter, J., Kumar, S., Zou, Y., Quintanilla, R., Sridharan, M., Carte, J., Chen, W., Roark, N., Ranganathan, S., Ravinder, N., & Chesnut, J. D. (2015). Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. Journal of Biotechnology, 208, 44–53. https://doi.org/10.1016/j.jbiotec.2015.04.024
Wang, M., Zuris, J. A., Meng, F., Rees, H., Sun, S., Deng, P., Han, Y., Gao, X., Pouli, D., Wu, Q., Georgakoudi, I., Liu, D. R., & Xu, Q. (2016). Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proceedings of the National Academy of Sciences of the United States of America, 113(11), 2868–2873. https://doi.org/10.1073/pnas.1520244113
Farboud, B., Jarvis, E., Roth, T. L., Shin, J., Corn, J. E., Marson, A., Meyer, B. J., Patel, N. H., & Hochstrasser, M. L. (2018). Enhanced genome editing with cas9 ribonucleoprotein in diverse cells and organisms. Journal of Visualized Experiments. https://doi.org/10.3791/57350
Jacków, J., Guo, Z., Hansen, C., Abaci, H. E., Doucet, Y. S., Shin, J. U., Hayashi, R., DeLorenzo, D., Kabata, Y., Shinkuma, S., Salas-Alanis, J. C., & Christiano, A. M. (2019). CRISPR/Cas9-based targeted genome editing for correction of recessive dystrophic epidermolysis bullosa using iPS cells. Proceedings of the National Academy of Sciences of the United States of America, 116(52), 26846–26852. https://doi.org/10.1073/pnas.1907081116
Zuris, J. A., Thompson, D. B., Shu, Y., Guilinger, J. P., Bessen, J. L., Hu, J. H., Maeder, M. L., Joung, J. K., Chen, Z. Y., & Liu, D. R. (2015). Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nature Biotechnology, 33(1), 73–80. https://doi.org/10.1038/nbt.3081
Paix, A., Folkmann, A., Rasoloson, D., & Seydoux, G. (2015). High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR-Cas9ribonucleoprotein complexes. Genetics, 201(1), 47–54. https://doi.org/10.1534/genetics.115.179382
Richardson, C. D., Ray, G. J., DeWitt, M. A., Curie, G. L., & Corn, J. E. (2016). Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nature Biotechnology, 34(3), 339–344. https://doi.org/10.1038/nbt.3481
Aida, T., Chiyo, K., Usami, T., Ishikubo, H., Imahashi, R., Wada, Y., Tanaka, K. F., Sakuma, T., Yamamoto, T., & Tanaka, K. (2015). Cloning-free CRISPR/Cas system facilitates functional cassette knock-in in mice. Genome Biology. https://doi.org/10.1186/s13059-015-0653-x
Kouranova, E., Forbes, K., Zhao, G., Warren, J., Bartels, A., Wu, Y., & Cui, X. (2016). CRISPRs for optimal targeting: Delivery of CRISPR components as DNA, RNA, and protein into cultured cells and single-cell embryos. Human Gene Therapy, 27(6), 464–475. https://doi.org/10.1089/hum.2016.009
Burger, A., Lindsay, H., Felker, A., Hess, C., Anders, C., Chiavacci, E., Zaugg, J., Weber, L. M., Catena, R., Jinek, M., Robinson, M. D., & Mosimann, C. (2016). Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes. Development (Cambridge), 143(11), 2025–2037. https://doi.org/10.1242/dev.134809
Sung, Y. H., Kim, J. M., Kim, H. T., Lee, J., Jeon, J., Jin, Y., Choi, J. H., Ban, Y. H., Ha, S. J., Kim, C. H., Lee, H. W., & Kim, J. S. (2014). Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Research, 24(1), 125–131. https://doi.org/10.1101/gr.163394.113
Wang, W., Kutny, P. M., Byers, S. L., Longstaff, C. J., DaCosta, M. J., Pang, C., Zhang, Y., Taft, R. A., Buaas, F. W., & Wang, H. (2016). Delivery of Cas9 protein into mouse zygotes through a series of electroporation dramatically increases the efficiency of model creation. Journal of Genetics and Genomics, 43(5), 319–327. https://doi.org/10.1016/j.jgg.2016.02.004
Hashimoto, M., Yamashita, Y., & Takemoto, T. (2016). Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non-mosaic mutants in the mouse. Developmental Biology, 418(1), 1–9. https://doi.org/10.1016/j.ydbio.2016.07.017
Chen, S., Lee, B., Lee, A. Y. F., Modzelewski, A. J., & He, L. (2016). Highly efficient mouse genome editing by CRISPR ribonucleoprotein electroporation of zygotes. Journal of Biological Chemistry, 291(28), 14457–14467. https://doi.org/10.1074/jbc.M116.733154
Kalebic, N., Taverna, E., Tavano, S., Wong, F. K., Suchold, D., Winkler, S., Huttner, W. B., & Sarov, M. (2016). CRISPR /Cas9-induced disruption of gene expression in mouse embryonic brain and single neural stem cells in vivo. EMBO Reports, 17(3), 338–348. https://doi.org/10.15252/embr.201541715
Mangeot, P. E., Risson, V., Fusil, F., Marnef, A., Laurent, E., Blin, J., Mournetas, V., Massouridès, E., Sohier, T. J. M., Corbin, A., Aubé, F., Teixeira, M., Pinset, C., Schaeffer, L., Legube, G., Cosset, F. L., Verhoeyen, E., Ohlmann, T., & Ricci, E. P. (2019). Genome editing in primary cells and in vivo using viral-derived Nanoblades loaded with Cas9-sgRNA ribonucleoproteins. Nature Communications. https://doi.org/10.1038/s41467-018-07845-z
Sun, W., Wang, J., Hu, Q., Zhou, X., Khademhosseini, A., & Gu, Z. (2020). CRISPR-Cas12a delivery by DNA-mediated bioresponsive editing for cholesterol regulation. Science Advances. https://doi.org/10.1126/sciadv.aba2983
Wan, T., Chen, Y., Pan, Q., Xu, X., Kang, Y., Gao, X., Huang, F., Wu, C., & Ping, Y. (2020). Genome editing of mutant KRAS through supramolecular polymer-mediated delivery of Cas9 ribonucleoprotein for colorectal cancer therapy. Journal of Controlled Release, 322, 236–247. https://doi.org/10.1016/j.jconrel.2020.03.015
Deng, S., Li, X., Liu, S., Chen, J., Li, M., Chew, S. Y., Leong, K. W., & Cheng, D. (2020). Codelivery of CRISPR-Cas9 and chlorin e6 for spatially controlled tumor-specific gene editing with synergistic drug effects. Science Advances. https://doi.org/10.1126/sciadv.abb4005
Azuma, H., Paulk, N., Ranade, A., Dorrell, C., Al-Dhalimy, M., Ellis, E., Strom, S., Kay, M. A., Finegold, M., & Grompe, M. (2007). Robust expansion of human hepatocytes in Fah-/-/Rag2 -/-/Il2rg-/- mice. Nature Biotechnology, 25(8), 903–910. https://doi.org/10.1038/nbt1326
Pankowicz, F. P., Barzi, M., Legras, X., Hubert, L., Mi, T., Tomolonis, J. A., Ravishankar, M., Sun, Q., Yang, D., Borowiak, M., Sumazin, P., Elsea, S. H., Bissig-Choisat, B., & Bissig, K. D. (2016). Reprogramming metabolic pathways in vivo with CRISPR/Cas9 genome editing to treat hereditary tyrosinaemia. Nature Communications, 7(1), 1–6. https://doi.org/10.1038/ncomms12642
Sun, W., Ji, W., Hall, J. M., Hu, Q., Wang, C., Beisel, C. L., & Gu, Z. (2015). Self-assembled DNA nanoclews for the efficient delivery of CRISPR-Cas9 for genome editing. Angewandte Chemie International Edition, 54(41), 12029–12033. https://doi.org/10.1002/anie.201506030
Sun, W., Jiang, T., Lu, Y., Reiff, M., Mo, R., & Gu, Z. (2014). Cocoon-like self-degradable DNA nanoclew for anticancer drug delivery. Journal of the American Chemical Society, 136(42), 14722–14725. https://doi.org/10.1021/ja5088024
Kim, Y.-K., Choi, J. Y., Jiang, H.-L., Arote, R., Jere, D., Cho, M.-H., Je, Y. H., & Cho, C.-S. (2009). Hybrid of baculovirus and galactosylated PEI for efficient gene carrier. Virology, 387(1), 89–97. https://doi.org/10.1016/j.virol.2009.02.001
Zhou, Z., Shen, Y., Tang, J., Jin, E., Ma, X., Sun, Q., Zhang, B., Van Kirk, E. A., & Murdoch, W. J. (2011). Linear polyethyleneimine-based charge-reversal nanoparticles for nuclear-targeted drug delivery. Journal of Materials Chemistry, 21(47), 19114–19123. https://doi.org/10.1039/c1jm13576g
Wei, Y., Gong, J., Thimmulappa, R. K., Kosmider, B., Biswal, S., & Duh, E. J. (2013). Nrf2 acts cell-autonomously in endothelium to regulate tip cell formation and vascular branching. Proceedings of the National Academy of Sciences of the United States of America, 110(41), E3910. https://doi.org/10.1073/pnas.1309276110
Denicola, G. M., Karreth, F. A., Humpton, T. J., Gopinathan, A., Wei, C., Frese, K., Mangal, D., Yu, K. H., Yeo, C. J., Calhoun, E. S., Scrimieri, F., Winter, J. M., Hruban, R. H., Iacobuzio-Donahue, C., Kern, S. E., Blair, I. A., & Tuveson, D. A. (2011). Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature, 475(7354), 106–110. https://doi.org/10.1038/nature10189
Zhou, G., Xu, Y., Chen, M., Cheng, D., & Shuai, X. (2016). Tumor-penetrating peptide modified and pH-sensitive polyplexes for tumor targeted siRNA delivery. Polymer Chemistry, 7(23), 3857–3863. https://doi.org/10.1039/c6py00427j
Pan, Y., Yang, J., Luan, X., Liu, X., Li, X., Yang, J., Huang, T., Sun, L., Wang, Y., Lin, Y., & Song, Y. (2019). Near-infrared upconversion-activated CRISPR-Cas9 system: A remote-controlled gene editing platform. Science Advances, 5(4), 7199. https://doi.org/10.1126/sciadv.aav7199
Guevara, M. L., Persano, F., & Persano, S. (2020). Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Frontiers in Chemistry. https://doi.org/10.3389/fchem.2020.589959
Tapeinos, C., Battaglini, M., & Ciofani, G. (2017). Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. Journal of Controlled Release. https://doi.org/10.1016/j.jconrel.2017.08.033
Barriga, H. M. G., Holme, M. N., & Stevens, M. M. (2019). Cubosomes: The next generation of smart lipid nanoparticles? Angewandte Chemie International Edition. https://doi.org/10.1002/anie.201804067
Shankar, R., Joshi, M., & Pathak, K. (2018). Lipid nanoparticles: A novel approach for brain targeting. Pharmaceutical Nanotechnology, 6(2), 81–93. https://doi.org/10.2174/2211738506666180611100416
Cunha, S., Amaral, M. H., Sousa Lobo, J. M., & Silva, A. C. (2017). Lipid nanoparticles for nasal/intranasal drug delivery. Critical Reviews in Therapeutic Drug Carrier Systems, 34(3), 257–282. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2017018693
Rajpoot, K. (2019). Solid lipid nanoparticles: A promising nanomaterial in drug delivery. Current Pharmaceutical Design, 25(37), 3943–3959. https://doi.org/10.2174/1381612825666190903155321
Samaridou, E., Heyes, J., & Lutwyche, P. (2020). Lipid nanoparticles for nucleic acid delivery: Current perspectives. Advanced Drug Delivery Reviews. https://doi.org/10.1016/j.addr.2020.06.002
ScioliMontoto, S., Muraca, G., & Ruiz, M. E. (2020). Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Frontiers in Molecular Biosciences. https://doi.org/10.3389/fmolb.2020.587997
Alavi, M., & Hamidi, M. (2019). Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metabolism and Personalized Therapy. https://doi.org/10.1515/dmpt-2018-0032
Fan, Y., Marioli, M., & Zhang, K. (2021). Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. Journal of Pharmaceutical and Biomedical Analysis. https://doi.org/10.1016/j.jpba.2020.113642
Chen, Z., Liu, F., Chen, Y., Liu, J. J., Wang, X., Chen, A. T., Deng, G., Zhang, H., Liu, J. J., Hong, Z., & Zhou, J. (2017). Targeted delivery of CRISPR/Cas9-mediated cancer gene therapy via liposome-templated hydrogel nanoparticles. Advanced Functional Materials, 27(46), 1703036. https://doi.org/10.1002/adfm.201703036
Cho, E. Y., Ryu, J. Y., Lee, H. A. R., Hong, S. H., Park, H. S., Hong, K. S., Park, S. G., Kim, H. P., & Yoon, T. J. (2019). Lecithin nano-liposomal particle as a CRISPR/Cas9 complex delivery system for treating type 2 diabetes. Journal of Nanobiotechnology. https://doi.org/10.1186/s12951-019-0452-8
Wei, T., Cheng, Q., Min, Y. L., Olson, E. N., & Siegwart, D. J. (2020). Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nature Communications, 11(1), 1–12. https://doi.org/10.1038/s41467-020-17029-3
Sugahara, K. N., Teesalu, T., Prakash Karmali, P., Ramana Kotamraju, V., Agemy, L., Greenwald, D. R., & Ruoslahti, E. (2010). Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science, 328(5981), 1031–1035. https://doi.org/10.1126/science.1183057
Gillmore, J. D., Gane, E., Taubel, J., Kao, J., Fontana, M., Maitland, M. L., Seitzer, J., O’Connell, D., Walsh, K. R., Wood, K., Phillips, J., Xu, Y., Amaral, A., Boyd, A. P., Cehelsky, J. E., McKee, M. D., Schiermeier, A., Harari, O., Murphy, A.,…Lebwohl, D. (2021). CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. New England Journal of Medicine, 385(6), 493–502. https://doi.org/10.1056/NEJMOA2107454
Gee, P., Lung, M. S. Y., Okuzaki, Y., Sasakawa, N., Iguchi, T., Makita, Y., Hozumi, H., Miura, Y., Yang, L. F., Iwasaki, M., Wang, X. H., Waller, M. A., Shirai, N., Abe, Y. O., Fujita, Y., Watanabe, K., Kagita, A., Iwabuchi, K. A., Yasuda, M.,…Hotta, A. (2020). Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nature Communications. https://doi.org/10.1038/s41467-020-14957-y
Rui, Y., Wilson, D. R., Choi, J., Varanasi, M., Sanders, K., Karlsson, J., Lim, M., & Green, J. J. (2019). Carboxylated branched poly(β-amino ester) nanoparticles enable robust cytosolic protein delivery and CRISPR-Cas9 gene editing. Science Advances. https://doi.org/10.1126/sciadv.aay3255
Zhou, W., Cui, H., Ying, L., & Yu, X. F. (2018). Enhanced cytosolic delivery and release of CRISPR/Cas9 by black phosphorus nanosheets for genome editing. Angewandte Chemie International Edition, 57(32), 10268–10272. https://doi.org/10.1002/anie.201806941
Montagna, C., Petris, G., Casini, A., Maule, G., Franceschini, G. M., Zanella, I., Conti, L., Arnoldi, F., Burrone, O. R., Zentilin, L., Zacchigna, S., Giacca, M., & Cereseto, A. (2018). VSV-G-enveloped vesicles for traceless delivery of CRISPR-Cas9. Molecular Therapy Nucleic Acids, 12, 453–462. https://doi.org/10.1016/j.omtn.2018.05.010
Lee, K., Conboy, M., Park, H. M., Jiang, F., Kim, H. J., Dewitt, M. A., Mackley, V. A., Chang, K., Rao, A., Skinner, C., Shobha, T., Mehdipour, M., Liu, H., Huang, W. C., Lan, F., Bray, N. L., Li, S., Corn, J. E., Kataoka, K.,…Murthy, N. (2017). Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nature Biomedical Engineering, 1(11), 889–901. https://doi.org/10.1038/s41551-017-0137-2
Chen, F., Alphonse, M., & Liu, Q. (2020). Strategies for nonviral nanoparticle-based delivery of CRISPR/Cas9 therapeutics. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. https://doi.org/10.1002/wnan.1609
Schmidt, F., & Grimm, D. (2015). CRISPR genome engineering and viral gene delivery: A case of mutual attraction. Biotechnology Journal. https://doi.org/10.1002/biot.201400529
Chamberlain, K., Riyad, J. M., & Weber, T. (2016). Expressing transgenes that exceed the packaging capacity of adeno-associated virus capsids. Human Gene Therapy Methods. https://doi.org/10.1089/hgtb.2015.140
Ramamoorth, M., & Narvekar, A. (2015). Non viral vectors in gene therapy—an overview. Journal of Clinical and Diagnostic Research. https://doi.org/10.7860/JCDR/2015/10443.5394
Li, L., Wei, Y., & Gong, C. (2015). Polymeric nanocarriers for non-viral gene delivery. Journal of Biomedical Nanotechnology. https://doi.org/10.1166/jbn.2015.2069
Jones, M. R., Seeman, N. C., & Mirkin, C. A. (2015). Programmable materials and the nature of the DNA bond. Science, 347(6224), 1260901–1260901. https://doi.org/10.1126/science.1260901
Macfarlane, R. J., Thaner, R. V., Brown, K. A., Zhang, J., Lee, B., Nguyen, S. B. T., & Mirkin, C. A. (2014). Importance of the DNA “bond” in programmable nanoparticle crystallization. Proceedings of the National Academy of Sciences of the United States of America, 111(42), 14995–15000. https://doi.org/10.1073/pnas.1416489111
Hu, R., Zhang, X., Zhao, Z., Zhu, G., Chen, T., Fu, T., & Tan, W. (2014). DNA nanoflowers for multiplexed cellular imaging and traceable targeted drug delivery. Angewandte Chemie, 126(23), 5931–5936. https://doi.org/10.1002/ange.201400323
Kim, H. J., Ishii, A., Miyata, K., Lee, Y., Wu, S., Oba, M., Nishiyama, N., & Kataoka, K. (2010). Introduction of stearoyl moieties into a biocompatible cationic polyaspartamide derivative, PAsp(DET), with endosomal escaping function for enhanced siRNA-mediated gene knockdown. Journal of Controlled Release, 145(2), 141–148. https://doi.org/10.1016/j.jconrel.2010.03.019
Miyata, K., Oba, M., Nakanishi, M., Fukushima, S., Yamasaki, Y., Koyama, H., Nishiyama, N., & Kataoka, K. (2008). Polyplexes from poly(aspartamide) bearing 1,2-diaminoethane side chains induce pH-selective, endosomal membrane destabilization with amplified transfection and negligible cytotoxicity. Journal of the American Chemical Society, 130(48), 16287–16294. https://doi.org/10.1021/ja804561g
Ding, Y., Jiang, Z., Saha, K., Kim, C. S., Kim, S. T., Landis, R. F., & Rotello, V. M. (2014). Gold nanoparticles for nucleic acid delivery. Molecular Therapy. https://doi.org/10.1038/mt.2014.30
Campbell, L. A., Coke, L. M., Richie, C. T., Fortuno, L. V., Park, A. Y., & Harvey, B. K. (2019). Gesicle-mediated delivery of CRISPR/Cas9 ribonucleoprotein complex for inactivating the HIV provirus. Molecular Therapy, 27(1), 151–163. https://doi.org/10.1016/j.ymthe.2018.10.002
Rui, Y., Wilson, D. R., Sanders, K., & Green, J. J. (2019). Reducible branched ester-amine quadpolymers (rBEAQs) codelivering plasmid DNA and RNA oligonucleotides enable CRISPR/Cas9 genome editing. ACS Applied Materials and Interfaces, 11(11), 10472–10480. https://doi.org/10.1021/acsami.8b20206
Wilson, D. R., Rui, Y., Siddiq, K., Routkevitch, D., & Green, J. J. (2019). Differentially branched ester amine quadpolymers with amphiphilic and pH-sensitive properties for efficient plasmid DNA delivery. Molecular Pharmaceutics, 16(2), 655–668. https://doi.org/10.1021/acs.molpharmaceut.8b00963
Gao, Y., Huang, J.-Y., O’Keeffe Ahern, J., Cutlar, L., Zhou, D., Lin, F.-H., & Wang, W. (2016). Highly branched poly(β-amino esters) for non-viral gene delivery: High transfection efficiency and low toxicity achieved by increasing molecular weight. Biomacromolecules, 17(11), 3640–3647. https://doi.org/10.1021/acs.biomac.6b01120
Armeanu, S., Pelisek, J., Krausz, E., Fuchs, A., Groth, D., Curth, R., Keil, O., Quilici, J., Rolland, P. H., Reszka, R., & Nikol, S. (2000). Optimization of nonviral gene transfer of vascular smooth muscle cells in vitro and in vivo. Molecular Therapy, 1(4), 366–375. https://doi.org/10.1006/mthe.2000.0053
Dokka, S., Toledo, D., Shi, X., Castranova, V., & Rojanasakul, Y. (2000). Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharmaceutical Research, 17(5), 521–525. https://doi.org/10.1023/A:1007504613351
Kim, K. K. E., Park, S. W., Kim, J. S. J. H. J. H., Lee, S. H., Kim, D., Koo, T., Kim, K. K. E., Kim, J. S. J. H. J. H., & Kim, J. S. J. H. J. H. (2017). Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Research, 27(3), 419–426. https://doi.org/10.1101/gr.219089.116
Noureddine, A., Maestas-Olguin, A., Saada, E. A., LaBauve, A. E., Agola, J. O., Baty, K. E., Howard, T., Sabo, J. K., Espinoza, C. R. S., Doudna, J. A., Schoeniger, J. S., Butler, K. S., Negrete, O. A., Brinker, C. J., & Serda, R. E. (2020). Engineering of monosized lipid-coated mesoporous silica nanoparticles for CRISPR delivery. Acta Biomaterialia, 114, 358–368. https://doi.org/10.1016/j.actbio.2020.07.027
Alton, E. W., Boyd, A. C., Porteous, D. J., Davies, G., Davies, J. C., Griesenbach, U., Higgins, T. E., Gill, D. R., Hyde, S. C., Innes, J. A., UK Cystic Fibrosis Gene Therapy Consortium. (2015). A phase I/IIa safety and efficacy study of nebulized liposome-mediated gene therapy for cystic fibrosis supports a multidose trial. American Journal of Respiratory and Critical Care Medicine. https://doi.org/10.1164/rccm.201506-1193LE
Zheng, P. P., Kros, J. M., & Li, J. (2018). Approved CAR T cell therapies: Ice bucket challenges on glaring safety risks and long-term impacts. Drug Discovery Today. https://doi.org/10.1016/j.drudis.2018.02.012
Shahryari, A., Jazi, M. S., Mohammadi, S., Nikoo, H. R., Nazari, Z., Hosseini, E. S., Burtscher, I., Mowla, S. J., & Lickert, H. (2019). Development and clinical translation of approved gene therapy products for genetic disorders. Frontiers in Genetics. https://doi.org/10.3389/fgene.2019.00868
Tuszynski, M. H. (2002). Growth-factor gene therapy for neurodegenerative disorders. The Lancet Neurology. https://doi.org/10.1016/S1474-4422(02)00006-6
Dickler, H. B., & Collier, E. (1994). Gene therapy in the treatment of disease. The Journal of Allergy and Clinical Immunology, 94(6 PART 1), 942–951. https://doi.org/10.1016/0091-6749(94)90111-2
Anderson, W. F. (1992). Human gene therapy. Science. https://doi.org/10.1126/science.1589762
Staahl, B. T., Benekareddy, M., Coulon-Bainier, C., Banfal, A. A., Floor, S. N., Sabo, J. K., Urnes, C., Munares, G. A., Ghosh, A., & Doudna, J. A. (2017). Efficient genome editing in the mouse brain by local delivery of engineered Cas9 ribonucleoprotein complexes. Nature Biotechnology, 35(5), 431–434. https://doi.org/10.1038/nbt.3806
Park, Y. J., Chang, L., Liang, J. F., Moon, C., Chung, C., & Yang, V. C. (2005). Nontoxic membrane translocation peptide from protamine, low molecular weight protamine (LMWP), for enhanced intracellular protein delivery: In vitro and in vivo study. The FASEB Journal, 19(11), 1555–1557. https://doi.org/10.1096/fj.04-2322fje
Kim, S. M., Shin, S. C., Kim, E. E., Kim, S. H., Park, K., Oh, S. J., & Jang, M. (2018). Simple in vivo gene editing via direct self-assembly of Cas9 ribonucleoprotein complexes for cancer treatment. ACS Nano, 12(8), 7750–7760. https://doi.org/10.1021/acsnano.8b01670
Wu, W., Lu, Z., Li, F., Wang, W., Qian, N., Duan, J., Zhang, Y., Wang, F., & Chen, T. (2017). Efficient in vivo gene editing using ribonucleoproteins in skin stem cells of recessive dystrophic epidermolysis bullosa mouse model. Proceedings of the National Academy of Sciences of the United States of America, 114(7), 1660–1665. https://doi.org/10.1073/pnas.1614775114
Wu, W., & Chen, T. (2019). Ribonucleoproteins mediated efficient in vivo gene editing in skin stem cells. Methods in molecular biology (Vol. 1879, pp. 75–86). New York: Humana Press Inc. https://doi.org/10.1007/7651_2018_115
Li, L., Hu, S., & Chen, X. (2018). Non-viral delivery systems for CRISPR/Cas9-based genome editing: Challenges and opportunities. Biomaterials. https://doi.org/10.1016/j.biomaterials.2018.04.031
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation for Research Centre for Medical Genetics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bykonya, A.G., Lavrov, A.V. & Smirnikhina, S.A. Methods for CRISPR-Cas as Ribonucleoprotein Complex Delivery In Vivo. Mol Biotechnol 65, 181–195 (2023). https://doi.org/10.1007/s12033-022-00479-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00479-z