Abstract
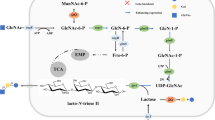
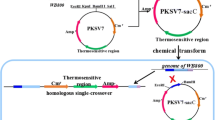
This study reports an alternative strategy for the expression of a recombinant l-AI from Enterococcus faecium DBFIQ E36 by auto-induction using glucose and glycerol as carbon sources and residual whey lactose as inducer agent. Commercial lactose and isopropyl β-d-1-thiogalactopyranoside (IPTG) were also evaluated as inducers for comparison of enzyme expression levels. The enzymatic extracts were purified by affinity chromatography, characterized, and applied in the bioconversion of d-galactose into d-tagatose. l-AI presented a catalytic activity of 1.67 ± 0.14, 1.52 ± 0.01, and 0.7 ± 0.04 U/mL, when expressed using commercial lactose, lactose from whey, and IPTG, respectively. Higher activities could be obtained by changing the protocol of enzyme extraction and, for instance, the enzymatic extract produced with whey presented a catalytic activity of 3.8 U/mL. The specific activity of the enzyme extracts produced using lactose (commercial or residual whey) after enzyme purification was also higher when compared to the enzyme expressed with IPTG. Best results were achieved when enzyme expression was conducted using 4 g/L of residual whey lactose for 11 h. These results proved the efficacy of an alternative and economic protocol for the effective expression of a recombinant l-AI aiming its high-scale production.








Similar content being viewed by others
References
Roy, S., Chikkerur, J., Roy, S. C., Dhali, A., Kolte, A. P., Sridhar, M., & Samanta, A. K. (2018). Tagatose as a potential nutraceutical: Production, properties, biological roles, and applications. Journal of Food Science, 83(11), 2699–2709. https://doi.org/10.1111/1750-3841.14358.
Guerrero-Wyss, M., Durán Agüero, S., & Angarita Dávila, L. (2018). d-Tagatose is a promising sweetener to control glycaemia: A new functional food. BioMed Research International. https://doi.org/10.1155/2018/8718053.
Mogha, K. V., Chaudhari, A. R., & Aparnathi, K. D. (2016). Tagatose: A low calorie multifunctional sweetener. Research and Reviews: Journal of Dairy Science and Technology, 5(3), 29–35.
Patra, F., Tomar, S. K., & Arora, S. (2009). Technological and functional applications of low-calorie sweeteners from lactic acid bacteria. Journal of Food Science, 74(1), 16–23. https://doi.org/10.1111/j.1750-3841.2008.01005.x.
Lu, Y., Levin, G. V., & Donner, T. W. (2008). Tagatose, a new antidiabetic and obesity control drug. Diabetes, Obesity and Metabolism, 10(2), 109–134. https://doi.org/10.1111/j.1463-1326.2007.00799.x.
Donner, T. W., Magder, L. S., & Zarbalian, K. (2010). Dietary supplementation with d-tagatose in subjects with type 2 diabetes leads to weight loss and raises high-density lipoprotein cholesterol. Nutrition Research, 30(12), 801–806. https://doi.org/10.1016/j.nutres.2010.09.007.
Levin, G. V. (2002). Tagatose, the new GRAS sweetener and health product. Journal of Medicinal Food, 5(1), 23–36. https://doi.org/10.1089/109662002753723197.
Kim, P. (2004). Current studies on biological tagatose production using l-arabinose isomerase: A review and future perspective. Applied Microbiology and Biotechnology, 65(3), 243–249. https://doi.org/10.1007/s00253-004-1665-8.
Mei, W., Wang, L., Zang, Y., Zheng, Z., & Ouyang, J. (2016). Characterization of an l-arabinose isomerase from Bacillus coagulans NL01 and its application for d-tagatose production. BMC Biotechnology. https://doi.org/10.1186/s12896-016-0286-5.
Yoon, S., Kim, P., & Oh, D. (2003). Properties of l-arabinose isomerase from Escherichia coli as biocatalyst for tagatose production. World Journal of Microbiology and Biotechnology, 19(1), 47–51. https://doi.org/10.1023/A:1022575601492.
Xu, Z., Qing, Y., Li, S., Feng, X., Xu, H., & Ouyang, P. (2011). A novel l-arabinose isomerase from Lactobacillus fermentum CGMCC2921 for d-tagatose production: Gene cloning, purification and characterization. Journal of Molecular Catalysis B: Enzymatic, 70(1–2), 1–7. https://doi.org/10.1016/j.molcatb.2011.01.010.
Manzo, R. M., Antunes, A. S. L. M., de Sousa Mendes, J., Hissa, D. C., Gonҫalves, L. R. B., & Mammarella, E. J. (2019). Biochemical characterization of heat-tolerant recombinant l-arabinose isomerase from Enterococcus faecium DBFIQ E36 strain with feasible applications in d-tagatose production. Molecular Biotechnology, 61(6), 385–399. https://doi.org/10.1007/s12033-019-00161-x.
Men, Y., Zhu, Y., Zhang, L., Kang, Z., Izumori, K., & Sun, Y. (2014). Enzymatic conversion of d-galactose to d-tagatose: Cloning, overexpression and characterization of L-arabinose isomerase from Pediococcus pentosaceus PC-5. Microbiological Research, 169(2–3), 171–178. https://doi.org/10.1016/j.micres.2013.07.001.
Liang, M., Chen, M., Liu, X., Zhai, Y., Liu, X., Zhang, H., et al. (2012). Bioconversion of d-galactose to d-tagatose: Continuous packed bed reaction with an immobilized thermostable l-arabinose isomerase and efficient purification by selective microbial degradation. Applied Microbiology and Biotechnology, 93(4), 1469–1474. https://doi.org/10.1007/s00253-011-3638-z.
Hung, X. G., Tseng, W. C., Liu, S. M., Tzou, W. S., & Fang, T. Y. (2014). Characterization of a thermophilic l-arabinose isomerase from Thermoanaerobacterium saccharolyticum NTOU1. Biochemical Engineering Journal, 83, 121–128. https://doi.org/10.1016/j.bej.2013.04.026.
Holsbeeck, M. V., Tsakali, E., Syryn, E., Aerts, G., Impe, J. V., Voorde, I. V., & De. . (2014). Overexpression of l-arabinose isomerase for production of the low-calorie bulk sweetener d-tagatose. Enzyme Engineering, 4(1), 1–6. https://doi.org/10.4172/2329-6674.1000125.
Xu, W., Fan, C., Zhang, T., Jiang, B., & Mu, W. (2016). Cloning, expression, and characterization of a novel l-arabinose isomerase from the psychrotolerant bacterium Pseudoalteromonas haloplanktis. Molecular Biotechnology, 58(11), 695–706. https://doi.org/10.1007/s12033-016-9969-3.
Machado, R., Azevedo-Silva, J., Correia, C., Collins, T., Arias, F. J., Rodríguez-Cabello, J. C., & Casal, M. (2013). High level expression and facile purification of recombinant silk-elastin-like polymers in auto induction shake flask cultures. AMB Express, 3(1), 11. https://doi.org/10.1186/2191-0855-3-11.
Rhimi, M., Ilhammami, R., Bajic, G., Boudebbouze, S., Maguin, E., Haser, R., & Aghajari, N. (2010). Bioresource technology the acid tolerant l-arabinose isomerase from the food grade Lactobacillus sakei 23K is an attractive d-tagatose producer. Bioresource Technology, 101(23), 9171–9177. https://doi.org/10.1016/j.biortech.2010.07.036.
Crowley, E. L., & Rafferty, S. P. (2019). Review of lactose-driven auto-induction expression of isotope-labelled proteins. Protein Expression and Purification, 157(January), 70–85. https://doi.org/10.1016/j.pep.2019.01.007.
Marbach, A., & Bettenbrock, K. (2012). lac operon induction in Escherichia coli: Systematic comparison of IPTG and TMG induction and influence of the transacetylase LacA. Journal of Biotechnology, 157(1), 82–88. https://doi.org/10.1016/j.jbiotec.2011.10.009.
Studier, F. W. (2005). Protein production by auto-induction in high-density shaking cultures. Protein Expression and Purification, 41(1), 207–234. https://doi.org/10.1016/j.pep.2005.01.016.
Dvorak, P., Chrast, L., Nikel, P. I., Fedr, R., Soucek, K., Sedlackova, M., et al. (2015). Exacerbation of substrate toxicity by IPTG in Escherichia coli BL21 ( DE3) carrying a synthetic metabolic pathway. Microbial Cell Factories. https://doi.org/10.1186/s12934-015-0393-3.
Malakar, P., & Venkatesh, K. V. (2012). Effect of substrate and IPTG concentrations on the burden to growth of Escherichia coli on glycerol due to the expression of lac proteins. Applied Microbiology and Biotechnology, 93(6), 2543–2549. https://doi.org/10.1007/s00253-011-3642-3.
Blommel, P. G., Becker, K. J., Duvnjak, P., & Fox, B. G. (2008). Enhanced bacterial protein expression during auto-induction obtained by alteration of lac repressor dosage and medium composition. Biotechnology Progress, 23(3), 585–598. https://doi.org/10.1021/bp070011x.
Briand, L., Marcion, G., Kriznik, A., Heydel, J. M., Artur, Y., Garrido, C., et al. (2016). A self-inducible heterologous protein expression system in Escherichia coli. Scientific Reports, 6(33037), 1–11. https://doi.org/10.1038/srep33037.
Guimarães, P. M. R., Teixeira, J. A., & Domingues, L. (2010). Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnology Advances, 28(3), 375–384. https://doi.org/10.1016/j.biotechadv.2010.02.002.
Vamvakaki, A. N., Kandarakis, I., Kaminarides, S., Komaitis, M., & Papanikolaou, S. (2010). Cheese whey as a renewable substrate for microbial lipid and biomass production by Zygomycetes. Engineering in Life Sciences, 10(4), 348–360. https://doi.org/10.1002/elsc.201000063.
Viitanen, M. I., Vasala, A., Neubauer, P., & Alatossava, T. (2003). Cheese whey-induced high-cell-density production of recombinant proteins in Escherichia coli. Microbial Cell Factories, 2(1), 2. https://doi.org/10.1186/1475-2859-2-2.
Manzo, R. M., Simonetta, A. C., Rubiolo, A. C., & Mammarella, E. J. (2013). Screening and selection of wild strains for l-arabinose isomerase production. Brazilian Journal of Chemical Engineering, 30(4), 711–720. https://doi.org/10.1590/S0104-66322013000400003.
Albuquerque, T. L., D’Almeida, A. P., Gomes, S. D. L., Fernandez-Lafuente, R., Gonçalves, L. R. B., & Rocha, M. V. P. (2018). Immobilization of β-galactosidase in glutaraldehyde-chitosan and its application to the synthesis of lactulose using cheese whey as feedstock. Process Biochemistry, 73, 65–73. https://doi.org/10.1016/j.procbio.2018.08.010.
Lima, A. F., Cavalcante, K. F., de Freitas, M., Rodrigues, T. H. S., Rocha, M. V. P., & Gonçalves, L. R. B. (2013). Comparative biochemical characterization of soluble and chitosan immobilized β-galactosidase from Kluyveromyces lactis NRRL Y1564. Process Biochemistry, 48(3), 443–452. https://doi.org/10.1016/j.procbio.2013.02.002.
Dische, Z., & Borenfreund, E. (1951). A new spectrophotometric method for the detection and determination of keto sugars and trioses. Journal of Biological Chemistry, 192, 583–587.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3.
Schägger, H., & Von Jagow, G. (1987). Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Analytical Biochemistry, 166, 368–379.
Fogler, H. S. (2009). Elementos de Engenharia das Reações Químicas (4th ed.). Rio de Janeiro: LTC: Livros Técnicos e Científicos Editora S.A.
Maier, U., & Büchs, J. (2001). Characterisation of the gas-liquid mass transfer in shaking bioreactors. Biochemical Engineering Journal, 7(2), 99–106. https://doi.org/10.1016/S1369-703X(00)00107-8.
Sousa, M. D., Manzo, R. M., Garc, L., Mammarella, E. J., Gonçalves, L. R. B., & Pessela, B. C. (2017). Engineering the l-arabinose isomerase from Enterococcus faecium for d-tagatose synthesis. Molecules, 22, 2164–2177. https://doi.org/10.3390/molecules22122164.
Cheng, L., Mu, W., & Jiang, B. (2010). Thermostable l-arabinose isomerase from Bacillus stearothermophilus IAM 11001 for d-tagatose production: Gene cloning, purification and characterisation. Journal of the Science of Food and Agriculture, 90(8), 1327–1333. https://doi.org/10.1002/jsfa.3938.
Salonen, N., Salonen, K., Leisola, M., & Nyyssölä, A. (2013). D-Tagatose production in the presence of borate by resting Lactococcus lactis cells harboring Bifidobacterium longuml-arabinose isomerase. Bioprocess and Biosystems Engineering, 36(4), 489–497. https://doi.org/10.1007/s00449-012-0805-2.
de Sousa, M., Silva Gurgel, B., Pessela, B. C., & Gonçalves, L. R. B. (2020). Preparation of CLEAs and magnetic CLEAs of a recombinant l-arabinose isomerase for d-tagatose synthesis. Enzyme and Microbial Technology. https://doi.org/10.1016/j.enzmictec.2020.109566.
Acknowledgements
The authors acknowledge the Brazilian Research Agencies CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), and FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico) for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
All authors approved the manuscript and this submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, T.C., Oliveira, R.C., Bezerra, S.G.S. et al. Alternative Heterologous Expression of l-Arabinose Isomerase from Enterococcus faecium DBFIQ E36 By Residual Whey Lactose Induction. Mol Biotechnol 63, 289–304 (2021). https://doi.org/10.1007/s12033-021-00301-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00301-2