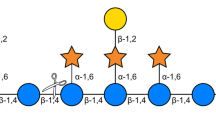
Abstract
Hemicellulose biomass is a complex polymer with many different chemical constituents that can be utilized as industrial feedstocks. These molecules can be released from the polymer and transformed into value-added chemicals through multistep enzymatic pathways. Some bacteria produce cellulosomes which are assemblies composed of lignocellulolytic enzymes tethered to a large protein scaffold. Rosettasomes are artificial engineered ring scaffolds designed to mimic the bacterial cellulosome. Both cellulosomes and rosettasomes have been shown to facilitate much higher rates of biomass hydrolysis compared to the same enzymes free in solution. We investigated whether tethering enzymes involved in both biomass hydrolysis and oxidative transformation to glucaric acid onto a rosettasome scaffold would result in an analogous production enhancement in a combined hydrolysis and bioconversion metabolic pathway. Three different enzymes were used to hydrolyze birchwood hemicellulose and convert the substituents to glucaric acid, a top-12 DOE value added chemical feedstock derived from biomass. It was demonstrated that colocalizing the three different enzymes to the synthetic scaffold resulted in up to 40 % higher levels of product compared to uncomplexed enzymes.





Similar content being viewed by others
References
Adsul, M. G., Singhvi, M. S., Gaikaiwari, S. A., & Gokhale, D. V. (2011). Development of biocatalysts for production of commodity chemicals from lignocellulosic biomass. Bioresource Technology, 102, 4304–4312.
Jordan, D. B., Bowman, M. J., Braker, J. D., Dien, B. S., Hector, R. E., Lee, C. C., et al. (2012). Plant cell walls to ethanol. Biochemical Journal, 442, 241–252.
Tuck, C. O., Pérez, E., Horváth, I. T., Sheldon, R. A., & Poliakoff, M. (2012). Valorization of biomass: deriving more value from waste. Science, 337, 695–699.
Juturu, V., & Wu, J. C. (2012). Microbial xylanases: engineering, production and industrial applications. Biotechnology Advances, 30, 1219–1227.
Lagaert, S., Pollet, A., Courtin, C. M., & Volckaert, G. (2014). β-Xylosidases and α-l-arabinofuranosidases: accessory enzymes for arabinoxylan degradation. Biotechnology Advances, 32, 316–332.
Zhang, Z., Donaldson, A. A., & Ma, X. (2012). Advancements and future directions in enzyme technology for biomass conversion. Biotechnology Advances, 30, 913–919.
Numan, M. T., & Bhosle, N. B. (2006). α-l-arabinofuranosidases: the potential applications in biotechnology. Journal of Industrial Microbiology and Biotechnology, 33, 247–260.
Poutanen, K., Tenkanen, M., Korte, H., & Puls, J. (1991). Accessory enzymes involved in the hydrolysis of xylans. In G. F. Leatham & M. E. Himmel (Eds.), Enzymes in biomass conversion (pp. 426–436). Washington, DC: American Chemical Society.
Buchala, A. J., & Wilkie, K. C. B. (1973). Total hemicelluloses from wheat at different stages of growth. Phytochemistry, 12, 499–505.
Buchala, A. J., & Wilkie, K. C. B. (1974). Total hemicelluloses from Hordeum vulgare plants at different stages of maturity. Phytochemistry, 13, 1347–1351.
Darvill, J. E., McNeil, M., Darvill, A. G., & Albersheim, P. (1980). Structure of plant cell walls: XI. Glucuronoarabinoxylan, a second hemicellulose in the primary cell walls of suspension-cultured sycamore cells. Plant Physiology, 66, 1135–1139.
Zhong, R., Pena, M. J., Zhou, G. K., Nairn, C. J., Wood-Jones, A., Richardson, E. A., et al. (2005). Arabidopsis fragile fiber8, which encodes a putative glucuronyltransferase, is essential for normal secondary wall synthesis. Plant Cell, 17, 3390–3408.
de Wet, B. J. M., & Prior, B. A. (2004). Microbial a-glucuronidases. In B. C. Saha & K. Hayashi (Eds.), Lignocellulose Biodegradation (pp. 241–254). Washington, DC: American Chemical Society.
Wagschal, K., Jordan, D. B., Lee, C. C., Younger, A., Braker, J. D., & Chan, V. J. (2015). Biochemical characterization of uronate dehydrogenases from three Pseudomonads, Chromohalobacter salixigens, and Polaromonas naphthalenivorans. Enyzme and Microbial Technology, 69, 62–68.
Boer, H., Maaheimo, H., Koivula, A., Penttila, M., & Richard, P. (2010). Identification in Agrobacterium tumefaciens of the d-galacturonic acid dehydrogenase gene. Applied Microbiology and Biotechnology, 86, 901–909.
Werpy, T., & Peterson, G. (2004). Top value added chemicals from biomass volume I—results of screening for potential candidates from sugars and synthesis gas. Oak Ridge, TN: US Department of Energy. http://www.nrel.gov/docs/fy04osti/35523.pdf.
Heerdt, A. S., Young, C. W., & Borgen, P. I. (1995). Calcium glucarate as a chemopreventive agent in breast cancer. Israel Journal of Medical Sciences, 31, 101–105.
Kiely, D. E., Chen, L., & Lin, T. H. (2000). Synthetic polyhydroxypolyamides from galactaric, xylaric, d-glucaric, and d-mannaric acids and alkylenediamine monomers—some comparisons. Journal of Polymer Science Polymer Chemistry, 38, 594–603.
Walaszek, Z., Hanausek, M., Narog, M., Raich, P. C., & Slaga, T. J. (2004). Mechanisms of lung cancer chemoprevention by d-glucarate. Chest, 125, 149S–150S.
Moon, T. S., Yoon, S. H., Lanza, A. M., Roy-Mayhew, J. D., & Prather, K. L. (2009). Production of glucaric acid from a synthetic pathway in recombinant Escherichia coli. Applied and Environment Microbiology, 75, 589–595.
Dueber, J. E., Wu, G. C., Malmirchegini, G. R., Moon, T. S., Petzold, C. J., Ullal, A. V., et al. (2009). Synthetic protein scaffolds provide modular control over metabolic flux. Nature Biotechnology, 27, 753–759.
Moon, T. S., Dueber, J. E., Shiue, E., & Prather, K. L. (2010). Use of modular, synthetic scaffolds for improved production of glucaric acid in engineered E. coli. Metabolic Engineering, 12, 298–305.
Bayer, E. A., Belaich, J. P., Shoham, Y., & Lamed, R. (2004). The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annual Review of Microbiology, 58, 521–554.
Fontes, C. M., & Gilbert, H. J. (2010). Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annual Review of Biochemistry, 79, 655–681.
Carvalho, A. L., Dias, F. M., Prates, J. A., Nagy, T., Gilbert, H. J., Davies, G. J., et al. (2003). Cellulosome assembly revealed by the crystal structure of the cohesin–dockerin complex. Proceedings of the National Academy of Sciences USA, 100, 13809–13814.
Schaeffer, F., Matuschek, M., Guglielmi, G., Miras, I., Alzari, P. M., & Beguin, P. (2002). Duplicated dockerin subdomains of Clostridium thermocellum endoglucanase CelD bind to a cohesin domain of the scaffolding protein CipA with distinct thermodynamic parameters and a negative cooperativity. Biochemistry, 41, 2106–2114.
Bhat, S., Goodenough, P. W., Bhat, M. K., & Owen, E. (1994). Isolation of four major subunits from Clostridium thermocellum cellulosome and their synergism in the hydrolysis of crystalline cellulose. International Journal of Biological Macromolecules, 16, 335–342.
Borne, R., Bayer, E. A., Pagès, S., Perret, S., & Fierobe, H. P. (2013). Unraveling enzyme discrimination during cellulosome assembly independent of cohesin–dockerin affinity. FEBS Journal, 280, 5764–5779.
Fierobe, H. P., Mechaly, A., Tardif, C., Belaich, A., Lamed, R., Shoham, Y., et al. (2001). Design and production of active cellulosome chimeras. Selective incorporation of dockerin-containing enzymes into defined functional complexes. Journal of Biological Chemistry, 276, 21257–21261.
Fierobe, H. P., Mingardon, F., Mechaly, A., Belaich, A., Rincon, M. T., Pages, S., et al. (2005). Action of designer cellulosomes on homogeneous versus complex substrates: controlled incorporation of three distinct enzymes into a defined trifunctional scaffoldin. Journal of Biological Chemistry, 280, 16325–16334.
McClendon, S. D., Mao, Z., Shin, H. D., Wagschal, K., & Chen, R. R. (2012). Designer xylanosomes: protein nanostructures for enhanced xylan hydrolysis. Applied Biochemistry and Biotechnology, 167, 395–411.
Morais, S., Morag, E., Barak, Y., Goldman, D., Hadar, Y., Lamed, R., et al. (2012). Deconstruction of lignocellulose into soluble sugars by native and designer cellulosomes. mBio, 3(6), e00508–12. doi:10.1128/mBio.00508-12.
Vazana, Y., Barak, Y., Unger, T., Peleg, Y., Shamshoum, M., Ben-Yehezkel, T., et al. (2013). A synthetic biology approach for evaluating the functional contribution of designer cellulosome components to deconstruction of cellulosic substrates. Biotechnology for Biofuels, 6, 182.
Mitsuzawa, S., Kagawa, H., Li, Y., Chan, S. L., Paavola, C. D., & Trent, J. D. (2009). The rosettazyme: a synthetic cellulosome. Journal of Biotechnology, 143, 139–144.
Paavola, C. D., Chan, S. L., Li, Y., Mazzarella, K. M., McMillan, R. A., & Trent, J. D. (2006). A versatile platform for nanotechnology based on circular permutation of a chaperonin protein. Nanotechnology, 17, 1171–1176.
Mishra, S., Beguin, P., & Aubert, J. P. (1991). Transcription of Clostridium thermocellum endoglucanase genes celF and celD. Journal of Bacteriology, 173, 80–85.
Lee, C. C., Smith, M., Kibblewhite-Accinelli, R. E., Williams, T. G., Wagschal, K., Robertson, G. H., & Wong, D. W. (2006). Isolation and characterization of a cold-active xylanase enzyme from Flavobacterium sp. Current Microbiology, 52, 112–116.
Lee, C. C., Kibblewhite, R. E., Wagschal, K., Li, R., & Orts, W. J. (2012). Isolation of α-glucuronidase enzyme from a rumen metagenomic library. Protein Journal, 31, 206–211.
Studier, F. W. (2005). Protein production by auto-induction in high density shaking cultures. Protein Expression and Purification, 41, 207–234.
Milner, Y., & Avigad, G. (1967). A copper reagent for the determination of hexuronic acids and certain ketohexoses. Carbohydrate Research, 4, 359–361.
Pell, G., Taylor, E. J., Gloster, T. M., Turkenburg, J. P., Fontes, C. M., Ferreira, L. M., et al. (2004). The mechanisms by which family 10 glycoside hydrolases bind decorated substrates. Journal of Biological Chemistry, 279, 9597–9605.
Kamat, R. K., Ma, W., Yang, Y., Zhang, Y., Wang, C., Kumar, C. V., & Lin, Y. (2013). Adsorption and hydrolytic activity of the polycatalytic cellulase nanocomplex on cellulose. ACS Appl Mater Interfaces, 5, 8486–8494.
Acknowledgements
We thank Bruce Mackey and Linda Whitehand for consultations on the statistical design of the experiments. This work was supported by the United States Department of Agriculture (CRIS 2030-41000-054-00) and National Institute of Food and Agriculture (Grant 2012-03998). The mention of firm names or trade products does not imply that they are endorsed or recommended by the US Department of Agriculture over other firms or similar products not mentioned. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, C.C., Kibblewhite, R.E., Paavola, C.D. et al. Production of Glucaric Acid from Hemicellulose Substrate by Rosettasome Enzyme Assemblies. Mol Biotechnol 58, 489–496 (2016). https://doi.org/10.1007/s12033-016-9945-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-016-9945-y