Abstract
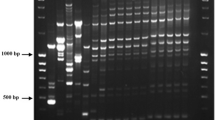
Colletotrichum capsici is an important fungal species that causes anthracnose in many genera of plants causing severe economic losses worldwide. A primer set was designed based on the sequences of the ribosomal internal transcribed spacer (ITS1 and ITS2) regions for use in a conventional PCR assay. The primer set (CcapF/CcapR) amplified a single product of 394 bp with DNA extracted from 20 Mexican isolates of C. capsici. The specificity of primers was confirmed by the absence of amplified product with DNA of four other Colletotrichum species and eleven different fungal genera. This primer set is capable of amplifying only C. capsici from different contaminated tissues or fungal structures, thereby facilitating rapid diagnoses as there is no need to isolate and cultivate the fungus in order to identify it. The sensitivity of detection with this PCR method was 10 pg of genomic DNA from the pathogen. This is the first report of a C. capsici-specific primer set. It allows rapid pathogen detection and provides growers with a powerful tool for a rational selection of fungicides to control anthracnose in different crops and in the post-harvest stage.





Similar content being viewed by others
References
Freeman, S. (2000). Genetic diversity and host specificity of Colletotrichum species on various fruits. In J. A. Bailey & M. J. Jegar (Eds.), Colletotrichum: Biology, pathology, control (pp. 57–77). Wallingfords: CAB International.
Bailey, J. A., & Jeger, M. J. (1992). Colletotrichum: Biology, pathology and control (p. 388). Wallingford: Commonwealth Mycological Institute.
Farr, D.F., Rossman, A.Y., Palm, M.E., & McCray, E.B. (2009) Fungal databases, Systematic Botany and Mycology Laboratory, ARS, USDA. Retrieved June 12, 2009, from http://nt.arsgrin.gov/fungaldatabases/new_allviewgenbank.cfm?thisName=Colletotrichum%20capsici&organismtype=Fungus.
Pakdeevaraporn, P., Wasee, S., Taylor, P. W. J., & Mongkolporn, O. (2005). Inheritance of resistance to anthracnose caused by Colletotrichum capsici in Capsicum. Plant Breeding, 124, 206–208.
Than, P. P., Jeewon, R., Hyde, K. D., Pongsupasamit, S., Mongkolporn, O., & Taylor, P. W. J. (2008). Characterization and pathogenicity of Colletotrichum species associated with anthracnose disease on chilli (Capsicum spp.) in Thailand. Plant Pathology, 57, 562–572.
Nantawanit, N., Chanchaichaovivat, A., Panijpan, B., & Ruenwongsa, P. (2010). Induction of defense response against Colletotrichum capsici in chili fruit by the yeast Pichia guillermondii strain R13. Biological Control, 52, 145–152.
Tapia-Tussell, R., Quijano-Ramayo, A., Cortes-Velazquez, A., Lappe, P., Larque-Saavedra, A., & Perez-Brito, D. (2008). PCR-based detection and characterization of the fungal pathogens Colletotrichum gloeosporioides and Colletotrichum capsici causing anthracnose in papaya (Carica papaya L.) in the Yucatan Peninsula. Molecular Biotechnology, 40, 293–298.
Tarnowski, T. L. B., & Ploetz, R. C. (2010). First report of Colletotrichum capsici causing postharvest anthracnose on papaya in South Florida. Plant Disease, 94, 1065.
Denoyes, B., & Baudry, A. (1995). Species identification and pathogenicity study of French Colletotrichum strains isolated from strawberry using morphological and cultural characteristics. Phytopathology, 85, 53–57.
Muñoz, J. A. G., Suarez, M. B., Grondona, I., Monte, E., Buddie, A. G., Bridge, P. D., et al. (2000). A physiological and biochemical approach to the systematics of Colletotrichum species pathogenic to strawberry. Mycologia, 92, 488–498.
Freeman, S., & Katan, T. (1997). Identification of Colletotrichum species responsible for anthracnose and root necrosis of strawberry in Israel. Phytopathology, 87, 516–521.
Martínez-Culebras, P. V., Barrio, E., García, M. D., & Querol, A. (2000). Identification of Colletotrichum species responsible for anthracnose of strawberry based on the internal transcribed spacers of the ribosomal region. FEMS Microbiology Letters, 189, 97–101.
Lewis, M. L., Nava-Diaz, C., & Miller, S. (2004). Identification and management of Colletotrichum acutatum on immature Bell Peppers. Plant Disease, 88, 1198–1204.
Freeman, S., Katan, T., & Shabi, E. (1996). Characterization of Colletotrichum gloeosporioides isolates from avocado and almond fruits with molecular and pathogenicity tests. Applied and Environmental Microbiology, 62, 1014–1020.
Peres, N. A. R., Souza, N. L., Zitko, S. E., & Timmer, L. W. (2002). Activity of benomyl for control of postbloom fruit drop citrus caused by Colletotrichum acutatum. Plant Disease, 86, 620–624.
Photita, W., Taylor, P. W. J., Ford, R., Hyde, K. D., & Lumyong, S. (2005). Morphological and molecular characterization of Colletotrichum species from herbaceous plants in Thailand. Fungal Diversity, 18, 117–133.
Goodwin, P. H., Hsiang, T., Xue, B. G., & Liu, H. W. (1995). Differentiation of Gaeumannomyces graminis from other turf-grass fungi by amplification with primers from ribosomal internal transcribed spacers. Plant Pathology, 44, 384–391.
Atkins, S. D., Clark, I. M., Pande, S., Hirsch, P. R., & Kerry, B. R. (2005). The use of real-time PCR and species-specific primers for the identification and monitoring of Paecilomyces lilacinus. FEMS Microbiology and Ecology, 51, 257–264.
Glen, M., Smith, A. H., Langrell, S. R. H., & Mohammed, C. L. (2007). Development of nested polymerase chain reaction detection of Mycosphaerella spp. and its application to the study of leaf disease in Eucalyptus plantations. Phytopathology, 97, 132–144.
Kikuchi, K., Matsushita, N., & Suzuki, K. (2007). Discrimination of Tricholoma species by species-specific ITS primers. Mycoscience, 48, 316–320.
Sreenivasaprasad, S., Sharada, K., Brown, A. E., & Mills, P. R. (1996). PCR-based detection of C. acutatum on strawberry. Plant Pathology, 45, 650–655.
Cullen, D. W., Lees, A. K., Toth, I. K., & Duncan, J. M. (2002). Detection of Colletotrichum coccodes from soil and potato tubers by conventional and quantitative real-time PCR. Plant Pathology, 51, 281–292.
Barnett, H. L., & Hunter, B. B. (1998). Illustrated genera of imperfect fungi, 4th edn. St. Paul: APS Press.
Tapia-Tussell, R., Lappe, P., Ulloa, M., Quijano-Ramayo, A., Cáceres-Farfán, M., Larqué-Saavedra, A., et al. (2006). A rapid and simple method for DNA extraction from yeasts and fungi isolated from Agave fourcroydes. Molecular Biotechnology, 33, 67–69.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Shinsky, & T. J. White (Eds.), PCR protocols: A guide to methods and applications (pp. 315–322). San Diego: Academic Press.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Rozen, S., & Skaletsky, H. J. (2000). Primer3 on the WWW for general users and for biologists programmers. In S. Krawetz & S. Misener (Eds.), Bioinformatics methods protocols: Methods in molecular biology (pp. 365–386). Totowa: Human Press.
Tapia-Tussell, R., Quijano-Ramayo, A., Rojas-Herrera, R., Larque-Saavedra, A., & Perez-Brito, D. (2005). A fast, simple, and reliable high-yielding method for DNA extraction from different plant species. Molecular Biotechnology, 31, 137–139.
Peres, N. A. R., Kuramae, E. E., Dias, M. S. C., & de Souza, N. L. (2002). Identification and Characterization of Colletotrichum spp. Affecting fruit after Harvest in Brazil. Journal of Phytopathology, 150, 128–134.
Chen, L. S., Chu, C., Liu, C. D., Chen, R. S., & Tsay, J. G. (2006). PCR-based detection and differentiation of Anthracnose Pathogens, Colletotrichum gloeosporioides and C. truncatum, from vegetable soybean in Taiwan. Journal of Phytopathology, 154, 654–662.
Wang, W., Tang, J. H., & Wang, Y. C. (2008). Molecular detection of Colletotrichum lindemuthianum by duplex PCR. Journal of Phytopathology, 156, 431–437.
Ippolito, A., Schena, L., Nigro, F., Ligorio, V. S., & Yaseen, T. (2004). Real-time detection of Phytophthora nicotianae and P. citrophthora in citrus roots and soil. European Journal of Plant Pathology, 110, 833–843.
Cia, P., Pascholati, S., Benato, E. A., Camili, E. C., & Santos, C. A. (2007). Effects of gamma and UV-C irradiation on the postharvest control of papaya anthracnose. Postharvest Biology and Technology, 43, 366–373.
Young, J. R., Tomaso-Peterson, M., Tredway, L. P., & de la Cerda, K. (2010). Occurrence and molecular identification of azoxystrobin-resistant Colletotrichum cereale isolates from golf course putting greens in the Southern United States. Plant Disease, 94, 751–757.
Acknowledgments
We thank Angel Nexticapan-Garcez for his help in samples collection. We are also grateful to Alberto Cortes-Velazquez and Anuar A. Magaña-Alvarez for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torres-Calzada, C., Tapia-Tussell, R., Quijano-Ramayo, A. et al. A Species-Specific Polymerase Chain Reaction Assay for Rapid and Sensitive Detection of Colletotrichum capsici . Mol Biotechnol 49, 48–55 (2011). https://doi.org/10.1007/s12033-011-9377-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-011-9377-7