Abstract
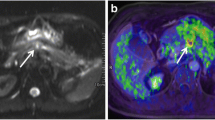
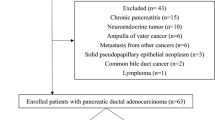
The aim of this prospective study was to investigate the accuracy and inter-observer reliability of MRI in detection of local recurrence (LR) of pancreatic adenocarcinoma (PAC) after surgery, which was proved by PET-CT and access correlation between functional MRI and PET parameters. Forty-five patients who underwent PET-CT and MRI for follow-up purposes after radical operation of PAC were included. Twenty-three were PET positive (study group) and 22 negative for LR (control group). MR examination was performed within one month after PET-CT and three readers who were blind for PET-CT findings searched LR in T2W, 3D-dynamic post-contrast T1W-FS and DWI sequences, respectively. Sensitivity and specificity were calculated while inter-reader agreement was estimated by Cronbach’s Alpha reliability coefficient (CARC). Apparent diffusion coefficient (ADC) of LR was correlated with the size (maximal diameter) and functional PET-CT parameters: mean and maximum standardized uptake values (SUVmean, SUVmax), metabolic tumor volume (MTV) and total lesion glycolysis (TLG), using Spearman’s correlation coefficient (rS). Sensitivity and specificity among three readers in detecting the LR were 70% and 77–84% in T2W (CARC 0.806), 91–100% and 100% in 3D post-contrast T1W-FS (CARC 0.980), and both 100% in DWI sequences (CARC 1.000). Moderate inverse correlation was found between the ADC and SUVmean (rS = − 0.484), MTV (rS = − 0.494), TLG (rS = − 0.519) and lesion size (rS = − 0.567). MRI with DWI shows high diagnostic accuracy in detecting the LR of PAC in comparison to PET-CT as reference standard. ADC significantly inversely correlates with standard and advanced PET parameters and size of LR.




Similar content being viewed by others
Data availability
Not applicable.
References
Siegel R, Ma J, Zou Z, et al. Cancer statistics. CA: Cancer J Clin. 2014;64(1):9–29. https://doi.org/10.3322/caac.21208.
Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2–11.
Shimada K, Sakamoto Y, Nara S, et al. Analysis of 5-year survivors after a macroscopic curative pancreatectomy for invasive ductal adenocarcinoma. World J Surg. 2010;34(8):1908–15.
Huicochea Castellanos S, Corrias G, Ulaner GA, et al. Detection of recurrent pancreatic cancer: value of second-opinion interpretations of cross-sectional images by subspecialized radiologists. Abdom Radiol. 2019;44(2):586–92.
Djuric-Stefanovic A, Gordanic N, Saponjski D, et al. Visualization of the fat planes between the pancreas and the adjacent organs and blood vessels using multi-detector computed tomography. Surg Radiol Anat. 2019. https://doi.org/10.1007/s00276-019-02214-x.
Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189(1):1–7.
Mortel KJ, Lemmerling M, de Hemptinne B, et al. Postoperative findings following the Whipple procedure: determination of prevalence and morphologic abdominal CT features. Eur Radiol. 2000;10(1):123–8.
Bluemke DA, Abrams RA, Yeo CJ, et al. Recurrent pancreatic adenocarcinoma: spiral CT evaluation following the Whipple procedure. Radiographics. 1997;17(2):303–13.
Kolbeinsson H, Hoppe A, Bayat A, et al. Recurrence patterns and postrecurrence survival after curative intent resection for pancreatic ductal adenocarcinoma. Surgery. 2021;169(3):649–54. https://doi.org/10.1016/j.surg.2020.06.042.
Elmi A, Murphy J, Hedgire S, et al. Post-Whipple imaging in patients with pancreatic ductal adenocarcinoma: association with overall survival: a multivariate analysis. Abdom Radiol. 2017;42(8):2101–7.
Kovač JD, Mayer P, Hackert T, et al. The time to and type of pancreatic cancer recurrence after surgical resection: is prediction possible. Acad Radiol. 2019. https://doi.org/10.1016/j.acra.2018.07.025.
Groot VP, Gemenetzis G, Blair AB, et al. Implications of the pattern of disease recurrence on survival following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2018. https://doi.org/10.1245/s10434-018-6558-7.
Djuric-Stefanovic A, Saponjski D, Mijovic K. The Cuff sign. Abdom Radiol. 2023;48:1862–4. https://doi.org/10.1007/s00261-023-03816-1.
Ergul N, Gundogan C, Tozlu M, et al. Role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in diagnosis and management of pancreatic cancer; comparison with multidetector row computed tomography, magnetic resonance imaging and endoscopic ultrasonography. Revi Esp Med Nucl Imagen Mol. 2014. https://doi.org/10.1016/j.remn.2013.08.005.
Sperti C, Pasquali C, Bissoli S, et al. Tumor relapse after pancreatic cancer resection is detected earlier by 18-FDG PET than by CT. J Gastrointest Surg. 2010. https://doi.org/10.1007/s11605-009-1010-8.
Hamidian Jahromi A, Sangster G, et al. Accuracy of multi-detector computed tomography, fluorodeoxyglucose positron emission tomography-CT, and CA 19–9 levels in detecting recurrent pancreatic adenocarcinoma. JOP J Pancreas. 2013;14(4):466–8.
Larson SM, Erdi Y, Akhurst T, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2(3):159–71.
Kang CM, Lee SH, Hwang HK, et al. Preoperative volume-based PET parameter, MTV25, as a potential surrogate marker for tumor biology and recurrence in resected pancreatic cancer. Medicine. 2016;95(9):e2595.
Heye T. CT diagnosis of recurrence after pancreatic cancer: is there a pattern? World J Gastroenterol. 2011;17(9):1126.
Balaj C, Ayav A, Oliver A, et al. CT imaging of early local recurrence of pancreatic adenocarcinoma following pancreaticoduodenectomy. Abdom Radiol. 2016;41(2):273–82.
Jung W, Jang J-Y, Kang MJ, et al. The clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography–computed tomography (PET–CT) in follow-up of curatively resected pancreatic cancer patients. HPB. 2016;18(1):57–64.
Yao X-Z, Yun H, Zeng M-S, et al. Evaluation of ADC measurements among solid pancreatic masses by respiratory-triggered diffusion-weighted MR imaging with inversion-recovery fat-suppression technique at 3.0T. Magn Reson Imaging. 2013;31(4):524–8.
Lee SS, Byun JH, Park BJ, et al. Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. J Magn Reson Imaging. 2008;28(4):928–36.
Barral M, Taouli B, Guiu B, et al. Diffusion-weighted MR imaging of the pancreas: current status and recommendations. Radiology. 2015;274(1):45–63.
Donswijk ML, Hess S, Mulders T, et al. [18F]Fluorodeoxyglucose PET/computed tomography in gastrointestinal malignancies. PET Clinics. 2014;9(4):421–41.
Lucignani G, Paganelli G, Bombardieri E. The use of standardized uptake values for assessing FDG uptake with PET in oncology: a clinical perspective. Nucl Med Commun. 2004;7:651–6.
Hamberg LM, Hunter GJ, Alpert NM, et al. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994;35(8):1308–12.
Wong CS, Gong N, Chu Y-C, et al. Correlation of measurements from diffusion weighted MR imaging and FDG PET/CT in GIST patients: ADC versus SUV. Eur J Radiol. 2012;9:2122–6.
Gu J, Khong P-L, Wang S, et al. Quantitative assessment of diffusion-weighted MR imaging in patients with primary rectal cancer: correlation with FDG-PET/CT. Mol Imag Biol. 2011;13(5):1020–8.
Ho K-C, Lin G, et al. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. Eur J Nucl Med Mol Imaging. 2009. https://doi.org/10.1007/s00259-008-0936-5.
Sakane M, Tatsumi M, Kim T, et al. Correlation between apparent diffusion coefficients on diffusion-weighted MRI and standardized uptake value on FDG-PET/CT in pancreatic adenocarcinoma. Acta Radiol. 2015;56(9):1034–41.
Huang WC, Sheng J, Chen SY, Lu JP. Differentiation between pancreatic carcinoma and mass-forming chronic pancreatitis: usefulness of high b value diffusion-weighted imaging. J Dig Dis. 2011;12(5):401–8.
Acknowledgements
“Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or European Education and Culture Executive Agency (EACEA). Neither the European Union nor the granting authority can be held responsible for them.” (EDUQUAN) ERASMUS-JMO-2021-HEI-TCH-RSCH.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saponjski, D., Djuric-Stefanovic, A., Jovanovic, M.M. et al. Utility of MRI in detection of PET-CT proven local recurrence of pancreatic adenocarcinoma after surgery. Med Oncol 41, 47 (2024). https://doi.org/10.1007/s12032-023-02271-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02271-8