Abstract
The presence of cancer stem cells (CSCs) in the tumor microenvironment (TME) is majorly responsible for the development and recurrence of cancer. Earlier reports suggested that upon DNA damage, poly-(ADP-ribose) polymerase-1 (PARP-1) helps in chromatin modulation and DNA repair process, thereby promoting CSC survival. But whether a combination of DNA damaging agents along with PARP inhibitors can modulate chromatin assembly, inhibit DNA repair processes, and subsequently target CSCs is not known. Hence, we have investigated the effect of nontoxic bioactive compound quinacrine (QC) and a potent PARP inhibitor Talazoparib in patient-derived oral mucosa CSCs (OM-CSCs) and in vivo xenograft mice preclinical model systems. Data showed that QC + Talazoparib inhibited the PARP-1-mediated chromatin remodelers’ recruitment and deregulated HAT activity of GCN5 (general control nonderepressible-5) and P300 at DNA damage site, thereby preventing the access of repair proteins to the damaged DNA. Additionally, this combination treatment inhibited topoisomerase activity, induced topological stress, and induced apoptosis in OM-CSCs. Similar results were observed in an in vivo xenograft mice model system. Collectively, the data suggested that QC + Talazoparib treatment inhibited BER pathway, induced genomic instability and triggered apoptosis in OM-CSCs through the deregulation of PARP-1-mediated chromatin remodelers (GCN5 and P300) activity.
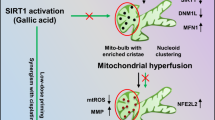
Graphical abstract
Schematic representation of QC + Talazoparib-induced apoptosis in oral mucosa CSCs. (1) Induction of DNA damage takes place after QC treatment (2) PARP1-mediated PARylation at the site of DNA damage, which recruits multiple chromatin remodelers (3) Acetylation at the histone tails relax the structure of chromatin and recruits the BER pathway proteins at the site of DNA damage. (4) BER pathway activated at the site of DNA damage. (5) CSCs survive after successful repair of DNA damage. (6) Treatment of QC-treated CSCs with PARP inhibitor Talazoparib (7) Inhibition of PARylation results in failure of chromatin remodelers to interact with PARP1. (8) Inhibition of acetylation status leads to chromatin compaction. (9) BER pathway proteins are not recruited at the site of DNA damage, resulting in inhibition of BER pathway and accumulation of unrepaired DNA damage, leading to apoptosis and cell death.







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- CSCs:
-
Cancer stem cells
- FBS:
-
Fetal bovine serum
- FDA:
-
Food and Drug Administration
- GCN5:
-
General control nonderepressible-5
- HAT:
-
Histone acetyltransferase
- OM-CSCs:
-
Oral mucosa cancer stem like cells
- PARP1:
-
Poly-(ADP-ribose) polymerase 1
- PBS:
-
Phosphate buffered saline
- PDX:
-
Patient-derived xenograft
- P-EMT:
-
Post-epithelial to mesenchymal transformed
- QC:
-
Quinacrine
- TME:
-
Tumor microenvironment
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Markopoulos AK. Current aspects on oral squamous cell carcinoma. Open Dent J. 2012;6:126.
Bhal S, Kundu CN. Targeting crosstalk of signaling pathways in cancer stem cells: a promising approach for development of novel anti-cancer therapeutics. Med Oncol. 2023;40:82.
Vinogradov S, Wei X. Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine. 2012;7:597–615.
Amjad MT, Chidharla A, Kasi A. Cancer chemotherapy. StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2023 [cited 2023 Apr 20]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK564367/.
Guo C, Gasparian AV, Zhuang Z, Bosykh DA, Komar AA, Gudkov AV, et al. 9-Aminoacridine-based anticancer drugs target the PI3K/AKT/mTOR, NF-κB and p53 pathways. Oncogene. 2009;28:1151–61.
Gurova K. New hopes from old drugs: revisiting DNA-binding small. Future Oncol. 2010;5:1–28.
Das B, Kundu CN. Anti-cancer stem cells potentiality of an anti-malarial agent quinacrine: an old wine in a new bottle. Anti-Cancer Agents Med Chem. 2021;21:416–27.
Preet R, Mohapatra P, Mohanty S, Sahu SK, Choudhuri T, Wyatt MD, et al. Quinacrine has anticancer activity in breast cancer cells through inhibition of topoisomerase activity. Int J Cancer. 2012;130:1660–70.
Preet R, Mohapatra P, Das D, Satapathy SR, Choudhuri T, Wyatt MD, et al. Lycopene synergistically enhances quinacrine action to inhibit Wnt-TCF signaling in breast cancer cells through APC. Carcinogenesis. 2013;34:277–86.
Nayak A, Satapathy SR, Das D, Siddharth S, Tripathi N, Bharatam PV, et al. Nanoquinacrine induced apoptosis in cervical cancer stem cells through the inhibition of hedgehog-GLI1 cascade: role of GLI-1. Sci Rep. 2016;6:1–16.
Völker-Albert M, Bronkhorst A, Holdenrieder S, Imhof A. Histone modifications in stem cell development and their clinical implications. Stem Cell Rep. 2020;15:1196–205.
Ghasemi S, Xu S, Nabavi SM, Amirkhani MA, Sureda A, Tejada S, et al. Epigenetic targeting of cancer stem cells by polyphenols (cancer stem cells targeting). Phytother Res. 2021;35:3649–64.
Sinha S, Molla S, Kundu CN. PARP1-modulated chromatin remodeling is a new target for cancer treatment. Med Oncol. 2021;38:1–16.
Zhang P, Torres K, Liu X, Liu C, Pollock RE. An overview of chromatin-regulating proteins in cells. Curr Protein Peptide Sci. 2016;17:401–10.
Trisciuoglio D, Di Martile M, Del Bufalo D. Emerging role of histone acetyltransferase in stem cells and cancer. Stem Cells Int. 2018;2018:8908751.
Guo P, Chen W, Li H, Li M, Li L. The histone acetylation modifications of breast cancer and their therapeutic implications. Pathol Oncol Res. 2018;24:807–13.
Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev. 2001;11:155–61.
Xu Y-M, Du J-Y, Lau AT. Posttranslational modifications of human histone H3: an update. Proteomics. 2014;14:2047–60.
Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–95.
Kuo Y-M, Andrews AJ. Quantitating the specificity and selectivity of Gcn5-mediated acetylation of histone H3. PLoS ONE. 2013;8: e54896.
Haque ME, Jakaria M, Akther M, Cho D-Y, Kim I-S, Choi D-K. The GCN5: its biological functions and therapeutic potentials. Clin Sci. 2021;135:231–57.
Iyer NG, Özdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23:4225–31.
Wang L, Chen K, Chen Z. Structural basis of ALC1/CHD1L autoinhibition and the mechanism of activation by the nucleosome. Nat Commun. 2021;12:1–9.
Qi W, Chen H, Lu C, Bu Q, Wang X, Han L. BRG1 promotes chromatin remodeling around DNA damage sites. Anim Cells Syst. 2018;22:360–7.
Wu T, Kamikawa YF, Donohoe ME. Brd4’s bromodomains mediate histone H3 acetylation and chromatin remodeling in pluripotent cells through P300 and Brg1. Cell Rep. 2018;25:1756–71.
Rose M, Burgess JT, O’Byrne K, Richard DJ, Bolderson E. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front Cell Dev Biol. 2020;8: 564601.
Paul S, Sinha S, Kundu CN. Targeting cancer stem cells in the tumor microenvironment: an emerging role of PARP inhibitors. Pharmacol Res. 2022;184:106425.
Molla S, Chatterjee S, Sethy C, Sinha S, Kundu CN. Olaparib enhances curcumin-mediated apoptosis in oral cancer cells by inducing PARP trapping through modulation of BER and chromatin assembly. DNA Repair. 2021;105: 103157.
Sinha S, Chatterjee S, Paul S, Das B, Dash SR, Das C, et al. Olaparib enhances the Resveratrol-mediated apoptosis in breast cancer cells by inhibiting the homologous recombination repair pathway. Exp Cell Res. 2022;420: 113338.
Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency BMN 673, a highly potent PARP inhibitor. Clin Cancer Res. 2013;19:5003–15.
FDA approves talazoparib for gBRCAm HER2-negative locally advanced or metastatic breast cancer. FDA [Internet]. 2019 [cited 2023 Mar 9]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-gbrcam-her2-negative-locally-advanced-or-metastatic-breast-cancer.
Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12.
Sethy C, Goutam K, Nayak D, Pradhan R, Molla S, Chatterjee S, et al. Clinical significance of a pvrl 4 encoded gene Nectin-4 in metastasis and angiogenesis for tumor relapse. J Cancer Res Clin Oncol. 2020;146:245–59.
Dash SR, Chatterjee S, Sinha S, Das B, Paul S, Pradhan R, et al. NIR irradiation enhances the apoptotic potentiality of quinacrine-gold hybrid nanoparticles by modulation of HSP-70 in oral cancer stem cells. Nanomed Nanotechnol Biol Med. 2022;40:102502.
Hembram KC, Dash SR, Das B, Sethy C, Chatterjee S, Bindhani BK, et al. Quinacrine based gold hybrid nanoparticles caused apoptosis through modulating replication fork in oral cancer stem cells. Mol Pharm. 2020;17:2463–72.
Nayak D, Tripathi N, Kathuria D, Siddharth S, Nayak A, Bharatam PV, et al. Quinacrine and curcumin synergistically increased the breast cancer stem cells death by inhibiting ABCG2 and modulating DNA damage repair pathway. Int J Biochem Cell Biol. 2020;119: 105682.
Pradhan R, Chatterjee S, Hembram KC, Sethy C, Mandal M, Kundu CN. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J Nutr Biochem. 2021;92: 108624.
Molla S, Hembram KC, Chatterjee S, Nayak D, Sethy C, Pradhan R, et al. PARP inhibitor olaparib enhances the apoptotic potentiality of curcumin by increasing the DNA damage in oral cancer cells through inhibition of BER cascade. Pathol Oncol Res. 2020;26:2091–103.
Das D, Preet R, Mohapatra P, Satapathy SR, Siddharth S, Tamir T, et al. 5-fluorouracil mediated anti-cancer activity in colon cancer cells is through the induction of adenomatous polyposis coli: implication of the long-patch base excision repair pathway. DNA Repair. 2014;24:15–25.
Sethy C, Kundu CN. PARP inhibitor BMN-673 induced apoptosis by trapping PARP-1 and inhibiting base excision repair via modulation of pol-β in chromatin of breast cancer cells. Toxicol Appl Pharmacol. 2022;436: 115860.
Karakaidos P, Karagiannis D, Rampias T. Resolving DNA damage: epigenetic regulation of DNA repair. Molecules. 2020;25:2496.
Audia JE, Campbell RM. Histone modifications and cancer. Cold Spring Harb Perspect Biol. 2016;8: a019521.
Ubhi T, Brown GW. Exploiting DNA replication stress for cancer treatmentexploiting replication stress. Can Res. 2019;79:1730–9.
Acknowledgements
The authors acknowledge Indian Council of Medical Research (ICMR), Government of India for providing research grant (F. No: 2016-0043/SCR/ADHOC-BMS) to CNK. The authors also thankful to Acharya Harihar Regional Cancer Centre (AHRCC), Cuttack, Odisha, India for providing patient sample for the experiments.
Funding
This work was supported by Indian Council of Medical Research (F. No: 2016-0043/SCR/ADHOC-BMS), Government of India.
Author information
Authors and Affiliations
Contributions
CD: Conceptualization, Methodology, Investigation, Validation, Visualization, Writing—original draft. SRD: Formal analysis, Validation, Visualization, Methodology, Writing—Review & Editing. SS: Formal analysis, Visualization, Methodology, Writing—Review & Editing. SP: Formal analysis, Validation, Resources, Methodology. BD: Validation, Visualization, Methodology. SB: Resources, Methodology. CS: Methodology. CNK: Conceptualization, Methodology, Investigation, Validation, Visualization, Project administration, Supervision, Funding acquisition, Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there are no conflicts of interest.
Ethical approval
All the patient tissue samples were used to carry out experiments by following the guidelines of the Declaration of Helsinki after getting the formal approval of the Institutional Ethics Committee (Ethical clearance approval letter No: 96-IEC-AHRCC) from Acharya Harihar Regional Cancer Centre, Cuttack, Odisha, India. Mice were used to carry out experiments by following the ARRIVE guidelines and the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India after receiving the formal approval (Ethical clearance approval Regd. #KSBT/IAEC/2022/MEET-1/A1) of Institutional Animal Ethics Committee (IAEC), KIIT University.
Consent for publication
All authors approve the submission of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, C., Dash, S.R., Sinha, S. et al. Talazoparib enhances the quinacrine-mediated apoptosis in patient-derived oral mucosa CSCs by inhibiting BER pathway through the modulation of GCN5 and P300. Med Oncol 40, 351 (2023). https://doi.org/10.1007/s12032-023-02222-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02222-3