Abstract
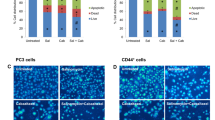
Cancer stem cells (CSCs) are associated with metastasis and recurrence in prostate cancer as well as other cancers. We aimed to enhance the sensitivity of cabazitaxel in prostate cancer cell therapy by targeting CSCs with a Wnt inhibitor and salinomycin pretreatment. PC3, DU-145, and LNCaP human prostate cancer cells were exposed to Wnt/β-catenin pathway inhibitor CCT036477 (iWnt) with salinomycin for 48 h, followed by cabazitaxel treatment for 48 h. Cell viability, mRNA, and protein expression changes were evaluated by MTT, RT-qPCR, and Western blot assays, respectively. Apoptosis was determined by image-based cytometry, and cell migration was assessed by wound healing assay. Three-dimensional culture was established to assess the malignant phenotype and stemness potential of transformed or cancer cells. CD44 + CSCs were isolated using magnetic-activated cell sorting system. Pretreatment of PC3, DU-145, and LNCaP cells with salinomycin iWnt significantly sensitized the cells to cabazitaxel therapy. Spheroid culture confirmed that the treatment modality was more effective than a single administration of chemotherapy. The pretreatment of PC3 cells increased the rate of apoptosis compared to single administration of cabazitaxel, which downregulated Bcl-2 and upregulated caspase 3, caspase 8 expressions. The pretreatment suppressed cell migration, downregulated the expression of Sox2 and Nanog, and significantly reduced CD44 + CSC numbers. Notably, the treatment modality reduced pAKT, p-P38 MAPK, and pERK1/2. The data suggest that pretreatment of prostate cancer cells with salinomycin and Wnt inhibitor may increase the efficacy of cabazitaxel therapy by inhibiting cell proliferation and migration, and eliminating cancer stem cells.
Graphical abstract








Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6(13):10697–711.
Yu EM, Aragon-Ching JB. Advances with androgen deprivation therapy for prostate cancer. Expert Opin Pharmacother. 2022;23(9):1015–33.
George DJ, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18(4):284–94.
Chen JR, et al. Comparison of systemic treatments for metastatic castration-resistant prostate cancer after docetaxel failure: a systematic review and network meta-analysis. Front Pharmacol. 2022. https://doi.org/10.3389/fphar.2021.789319.
Alfarouk KO, et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71–71.
Ritch C, Cookson M. Recent trends in the management of advanced prostate cancer. F1000Res. 2018;7:1513.
Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781.
Takebe N, et al. Targeting notch, hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12(8):445–64.
Anadón A, Martínez-Larrañaga MR. Veterinary drugs residues: coccidiostats. In: Motarjemi Y, editor. Encyclopedia of food safety. Waltham: Academic Press; 2014. p. 63–75.
Kim JH, et al. Salinomycin sensitizes cancer cells to the effects of doxorubicin and etoposide treatment by increasing DNA damage and reducing p21 protein. Br J Pharmacol. 2011;162(3):773–84.
Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–59.
Yu J, et al. Salinomycin triggers prostate cancer cell apoptosis by inducing oxidative and endoplasmic reticulum stress via suppressing Nrf2 signaling. Exp Ther Med. 2021;22(3):946.
Versini A, et al. Salinomycin derivatives kill breast cancer stem cells by lysosomal iron targeting. Chemistry. 2020;26(33):7416–24.
Qi D, et al. Salinomycin as a potent anticancer stem cell agent: state of the art and future directions. Med Res Rev. 2022;42(3):1037–63.
Erdogan S, et al. The synergistic anticancer effect of salinomycin combined with cabazitaxel in CD44+prostate cancer cells by downregulating wnt, NF-kappa B and AKT signaling. Mol Biol Rep. 2022;49(6):4873–84.
Ng LF, et al. WNT signaling in disease. Cells. 2019;8(8):826.
Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165.
Borah A, et al. Targeting self-renewal pathways in cancer stem cells: clinical implications for cancer therapy. Oncogenesis. 2015;4:e177.
Ewan K, et al. A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription. Cancer Res. 2010;70(14):5963–73.
Fu SF, et al. N-methyl-N-nitrosourea induces zebrafish anomalous angiogenesis through Wnt/β-Catenin pathway. Ecotoxicol Environ Saf. 2022;239:113674.
Fronk AH, Vargis E. Methods for culturing retinal pigment epithelial cells: a review of current protocols and future recommendations. J Tissue Eng. 2016;7:2041731416650838.
Oka MS, Landers RA, Bridges CD. A serum-free defined medium for retinal pigment epithelial cells. Exp Cell Res. 1984;154(2):537–47.
Su CY, et al. Analyzing the expression of biomarkers in prostate cancer cell lines. In Vivo. 2021;35(3):1545–8.
Erdogan S, Turkekul K. Neferine inhibits proliferation and migration of human prostate cancer stem cells through p38 MAPK/JNK activation. J Food Biochem. 2020. https://doi.org/10.1111/jfbc.13253.
Han SJ, Kwon S, Kim KS. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021;21(1):152.
Ketola K, et al. Salinomycin inhibits prostate cancer growth and migration via induction of oxidative stress. Br J Cancer. 2012;106(1):99–106.
Liu Q, et al. Salinomycin suppresses tumorigenicity of liver cancer stem cells and Wnt/Beta-catenin signaling. Curr Stem Cell Res Ther. 2021;16(5):630–7.
Tyagi M, Patro BS. Salinomycin reduces growth, proliferation and metastasis of cisplatin resistant breast cancer cells via NF-kB deregulation. Toxicol In Vitro. 2019;60:125–33.
Liu L, et al. Salinomycin suppresses cancer cell stemness and attenuates TGF-beta-induced epithelial-mesenchymal transition of renal cell carcinoma cells. Chem Biol Interact. 2018;296:145–53.
Barbato L, et al. Cancer stem cells and targeting strategies. Cells. 2019;8(8):926.
Patni AP, et al. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma-clinical implications. Cell Oncol. 2021;44(3):473–94.
Klose J, et al. Salinomycin: anti-tumor activity in a pre-clinical colorectal cancer model. PLoS ONE. 2019;14(2):e0211916.
Gehrke T, et al. Combination of salinomycin and radiation effectively eliminates head and neck squamous cell carcinoma cells in vitro. Oncol Rep. 2018;39(4):1991–8.
Arafat K, et al. Inhibitory effects of salinomycin on cell survival, colony growth, migration, and invasion of human non-small cell lung cancer A549 and LNM35: involvement of NAG-1. PLoS ONE. 2013;8(6):e66931.
Dewangan J, et al. Salinomycin inhibits breast cancer progression via targeting HIF-1alpha/VEGF mediated tumor angiogenesis in vitro and in vivo. Biochem Pharmacol. 2019;164:326–35.
Raisova M, et al. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117(2):333–40.
Kim KY, et al. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int J Mol Sci. 2017;18(5):1088.
Kim KY, et al. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem Biophys Res Commun. 2011;413(1):80–6.
Lee SH, et al. Androgen signaling is a confounding factor for beta-catenin-mediated prostate tumorigenesis. Oncogene. 2016;35(6):702–14.
Wang F, et al. Wnt/beta-catenin signaling contributes to prostate cancer heterogeneity through reciprocal suppression of H3K27 trimethylation. Biochem Biophys Res Commun. 2020;527(1):242–9.
Bian P, et al. Activated Wnt/f3-catenin signaling contributes to E3 ubiquitin ligase EDD-conferred docetaxel resistance in prostate cancer. Life Sci. 2020;254:116816.
Fang F, et al. CD147 promotes epithelial-mesenchymal transition of prostate cancer cells via the Wnt/beta-catenin pathway. Exp Ther Med. 2020;20(4):3154–60.
Liao JQ, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE. 2014;9(1):e84941.
Lee HG, et al. Salinomycin reduces stemness and induces apoptosis on human ovarian cancer stem cell. J Gynecol Oncol. 2017. https://doi.org/10.3802/jgo.2017.28.e14.
Yang L, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8.
Zhang Y, et al. Bortezomib potentiates antitumor activity of mitoxantrone through dampening Wnt/beta-catenin signal pathway in prostate cancer cells. BMC Cancer. 2021. https://doi.org/10.1186/s12885-021-08841-1.
Yu X, et al. Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30(16):1868–79.
Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24(6):1838–51.
Wang W, et al. Down-regulation of E-cadherin enhances prostate cancer chemoresistance via Notch signaling. Chin J Cancer. 2017;36(1):35.
Witta SE, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66(2):944–50.
Shang Z, et al. A switch from CD44(+) cell to EMT cell drives the metastasis of prostate cancer. Oncotarget. 2015;6(2):1202–16.
Qu H, et al. Effect of salinomycin on metastasis and invasion of bladder cancer cell line T24. Asian Pac J Trop Med. 2015;8(7):578–82.
Acknowledgements
We would like to thank Drs. Ruveyde Garip and Sultan Kaya (Trakya University, School of Medicine, Department of Ophthalmology) for their support in the preparation of bovine retinal pigment epithelial cells.
Funding
This study was funded by the Trakya University Research Project Foundation (Project no: 2020-01), Edirne, Turkey.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Experiments were performed and analyzed by Riza Serttas. The first draft of the manuscript was written by Suat Erdogan and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial, personal, or professional conflict of interest.
Ethical approval
Not applicable. This article does not contain any studies with human participants or live animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Serttas, R., Erdogan, S. Pretreatment of prostate cancer cells with salinomycin and Wnt inhibitor increases the efficacy of cabazitaxel by inducing apoptosis and decreasing cancer stem cells. Med Oncol 40, 194 (2023). https://doi.org/10.1007/s12032-023-02062-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02062-1