Abstract
Triple negative breast cancer (TNBC) portraying deficient expression of estrogen receptor (ER), progesterone receptor (PR) and Human epidermal growth factor receptor 2 (HER2) is known to be the most aggressive subtype associated with poor prognosis and interventional strategies limited to chemotherapy and breast conserving surgery. Some TNBC incidences have also been reported with positive circ-HER2 expression thus rendering circ-HER2 a potential immunotherapy target to direct drug development. Resistance and recurrence reported with traditional approaches has led us towards the application of immunotherapeutic interventions owing to their anti-tumor efficacy. This review provides an elaborative insight on potential molecular biomarkers to be targeted by immunotherapy. Additionally, clinical trials proposing the application of immunotherapy in neoadjuvant, adjuvant and metastatic TNBC setting have also been included. The gathered evidence indicates a positive application of immunotherapy in TNBC with therapeutic limitation available only owing to the possibility of adverse events which can be dealt considering risk-to-benefit ratio. Furthermore, potential targets to aim for therapeutic vaccines along with evidence from clinical trials have also been mentioned.
Similar content being viewed by others
Introduction
Breast cancer (BC) forms one of the most common culprits of invasive cancers in women. BC had the highest frequency of diagnosis in women worldwide with case counts rising to 2.26 million, compared to 0.41 million in uterine, 0.6 million in cervical and 0.3 million in ovarian cancers in the year 2020 [1]. The incidence of BC has been higher in developed countries and this finding is contributed by the fact that women in developed countries give fewer births and breastfeed for shorter durations, as compared to the developing ones [2]. Though the developed countries stood foremost in incidence rates, countries located in Asia and Africa shared 63% of the total death count in 2020 [3]. Survival was better in high-income and developed countries as compared to low income and many middle-income countries [4]. Over the last three decades incidence and death rates have remarkably increased, for which population structure, environment, genetic particulars and quality of life could be few of the responsible factors [5, 6].
BC encompasses wide phenotypical heterogeneity due to which the clinical profile of each of its subtype varies as they possess distinct demeanors towards the therapy [7]. Several molecular biomarkers are defined under the pathology of this cancer such as estrogen receptor alpha-positive (ERα+), progesterone receptor-positive (PR+), human epidermal growth factor receptor-2 (HER-2/ERBB2), epidermal growth factor receptor (EGFR), Cytokeratin 5/6 (CK5/6), vascular endothelial growth factor (VEGF) and Antigen KI67 [8]. Identification of subtypes with distinguishing prognosis and therapy targets of BC stems from gene expression studies [9]. Differing immunohistochemical properties aid classification of this carcinoma into five namely Luminal A, Luminal B, HER2, Triple negative and normal-like breast cancer. The prognosis profile ranges from best for Luminal A type to worst for triple negative breast cancer (TNBC). The prevalence rates are hereby given in a descending order with the highest seen in Luminal A(70%) followed by Triple negative (15–20%), Luminal B (10–20%), HER2 (5–15%) with least rate observed in normal-like breast cancer [10]. The above statements lead us to the fact that TNBC has the worst prognostication accompanying its higher occurrence. Ergo, embarking upon TNBC, it gained its substantiality in the mid-2000s. The poor prognosis of TNBC is owed to the lack of estrogen and progesterone receptors and under-expression of HER-2 which impedes achievement of successful treatment outcomes [11]. There exists four subtypes of TNBC based on the cellular particulars that include basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal (M) and luminal androgen receptor (LAR). These four TNBC subtypes are associated with distinct expression patterns with immune-modulatory infiltrates varying within each of these subtypes [12]. For further advancements in the development of successful treatment strategies, confirmatory prognostic and predictive biomarkers are desired [13].
The Traditional therapy aiming to mitigate TNBC includes Neoadjuvant therapy, Adjuvant therapy, Surgery and Radiotherapy. Contraction of TNBC using the conventional approach faced a major shortcoming of resistance accompanied by numerous side effects and this demanded the advent of a newer perspective to treat this carcinoma. Aiming to develop even more precise intervention and eradicate the limitations mentioned formerly, immunotherapy was introduced to deal with the carcinogenesis with a novel vision [14]. Immunotherapy strengthens the resident immune machinery and prepares it to recognize and destroy cancerous cells with enhanced efficiency [15]. The immunological profile of TNBC portrays a tumor microenvironment (TME) with abundant lymphocyte infiltration and overexpression of Programmed Death-Ligand 1 (PD-L1) as compared to other subtypes [16]. In addition, TNBC presents with a higher number of somatic mutations resulting from genomic instability which escalates the frequency of neoantigen availability [17]. This depicts a higher responding capability of TNBC to immunotherapy in contrast to traditional approaches. Classes that are conventionally established on the foundation of immunotherapy include monoclonal antibodies, checkpoint inhibitors, cytokines, vaccines and Chimeric Antigen Receptor-T (CAR-T) cell therapy. Monoclonal antibodies offer target specificity with low toxicity profiles. They work in one or multiple ways depending on the antigen its targeting. Checkpoint inhibitors on the other hand target specific immune checkpoint proteins that regulate the functioning of immune system [18]. Cytokine inhibitors target cytokines which are highly inducible secretory proteins that mediate the communication between the immune cells [19]. Onco-vaccines can be preventive or therapeutic that mainly use tumor associated antigens (TAAs) and tumor specific antigens (TSAs). CAR-T cell therapy uses techniques to modify T-cells to produce chimeric antigen receptors. These receptors allow T-cells to get attached to the desired antigens [20]. This review focuses on revealing additional information regarding immunotherapy and to define the targets along with the target-specific interventions for TNBC.
Molecular targets of immunotherapy for breast cancer
The pathogenesis of BC unveils contribution of numerous molecular targets in uncontrolled proliferation and promotion of survival of the tumor. Identification of these molecular targets present a lead towards development of potential drugs that rectify the overexpression of such biomarkers and help eradicate the disease [21]. The potency of these molecular targets and the drugs aimed for achieving anti-tumor activity in TNBC are presented in Fig. 1. This list of molecular targets extends from mesothelin, CD133, CTLA4, PD-1/PD-L1, LAG-3, STAT-3, IDO to CD3 and HER2 and is summarized in Table 1.
Molecular targets holding clinical potential to aim immunotherapy interventions for their immunomodulatory effect. (Tumor-infiltrating lymphocyte (TIL), antigen-presenting cell (APC), programmed death-1 (PD-1), programmed death ligand-1 (PD-L1), cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4), regulatory T- cells (Treg cells), indoleamine 2,3-dioxygenase (IDO), tryptophan (Trp), kynurenine (Kyn), T-cell receptor (TCR), L-tryptophan (L-TRP))
Mesothelin (MSLN), a 40 kDa glycosyl-phosphatidyl inositol-linked membrane glycoprotein is expressed on the mesothelial cells surfaced on peritoneum, pericardium and pleura. Overexpression of MSLN factors in progression of cancers presenting with aggressive phenotypical characteristics and poorer prognosis [22]. It is a key component in sustaining the course of progression, invasion and survival of tumor cells besides developing drug resistance. This overexpression has recently been demonstrated in TNBC thus making it a potential molecular site for targeting therapeutic interventions [23].
CD133 otherwise known as prominin-1 is a pentaspan transmembrane single chain glycoprotein residing in the protrusions present in the biological membrane with specific availability in cholesterol-based lipid domains [24]. Due to its availability on surface, it qualifies as a surface marker for the detection of cancer stem cells [25]. Mislocalization of CD133 from surface to nucleus disrupts transcriptional regulation by causing an interference in the molecular cascades directly in association with proliferation and differentiation of cancerous cells [26]. This deduces CD133 as a potential target to eradicate disease pathology.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) is a glycoprotein notably expressed on the cell surface of stimulated T cells, subset of Tregs and on non-lymphoid cells of different tissues alongside being expressed on the surface of some solid tumors [27]. CTLA-4 has a potential to down-regulate T-cell responses and fade peripheral tolerance which shrinks the efficacy of generated antitumor response ultimately leading to tumor immune tolerance [28]. Upregulation of CTLA-4 is one of the important underlying causes of immune evasion and this addresses the role of anti-CTLA-4 antibodies in acquiring CTLA-4 as a putative drug target to obtain optimal therapeutic action [29]. Tremelimumab and Ipilimumab drugs are both fully humanized monoclonal antibodies capable of inducing durable responses by blocking CTLA-4 antigen [30].
PD-1/PD-L1 is a programmed death-1 receptor-ligand system, also referred to as CD279 and B7-H1 is a 33-kDa type 1 transmembrane glycoprotein usually expressed on activated T cells, natural killer (NK) cells, B lymphocytes, macrophages, dendritic cells (DCs) and monocytes with higher level of expression on tumor specific T-cells [31]. PD-1 has a negative impact on adaptive and innate immune responses down-regulating antitumor responses. It inhibits proliferation of tumor-infiltrating lymphocytes, halts cytokine production and strengthens tumor to escape immune response generated by antibodies, ultimately favouring tumor survival [32]. This suggests that it is imperative to target drugs on PD-1/PD-L1 to reverse its detrimental effect. Pembrolizumab, Avelumab, Atezolizumab and Nivolumab are monoclonal antibodies with a proposed mechanism of preventing the interaction of PD-1 receptor with PD-L1 ligand to diminish the consequences of PD-1 pathway-mediated immune responses against tumor cells [33]. As this chain of mechanistic cascades also function in TNBC, the role of these drugs can be explored in diminishing the influence of this carcinoma.
Lymphocyte activation gene-3 (LAG-3) alternatively addressed as CD223 is expressed on the surface of T cells, NK cells, NK T cells and regulatory T (Treg) cells [34]. Overexpression of LAG-3 translates into negative regulation of T cell stimulation and proliferation [35]. It further may also yield a synergistic effect with PD-1/PD-L1 to facilitate depletion of immunity [36]. This ultimately worsens the course of carcinogenesis and categorizes itself as one of the immune checkpoints to target by developing drugs to accomplish superiority over tumorigenesis. LAG-3, CTLA-4 and PD-1/PD-L1 inhibitory drugs are housed under the drug class of immune checkpoint inhibitors (ICIs).
Signal transducer and activator of transcription 3 (STAT3) are designated to be an early tumor diagnostic biomarker localized in basal-like cells of breast cancer cells CD44+ CD24– [37]. Constitutive activation and overexpression of STAT3 upregulate cyclin D-1, c-myc, and bcl-2 and suppress the apoptosis of tumor cells promoting tumor survival and progression [38]. Thus, it is worthy to note STAT3 as one of the probable biomarkers to aim for during drug development process.
Indoleamine-2,3-dioxygenase (IDO) is an immunosuppressive enzyme expressed by dendritic cells residing in tissues and draining lymph nodes of breast cancer patients. IDO catabolizes the breakdown of an essential amino acid L-tryptophan into kynurenines which causes L-tryptophan deficiency [39]. Such a deficient reserve of amino acid exhausts the cytotoxicity of T cells disabling their capability to withstand the negative influence of tumor progression [40]. Therefore, IDO enzyme inhibitors evolve as an approach to consider for immunotherapy development towards TNBC.
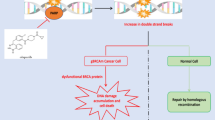
HER2 is 180 kDa transmembrane glycoprotein present on the surface of diverse set of T cells whose overexpression is associated with promotion of malignancy of cancer by generating anti-apoptotic signals [41]. TNBC lack expression of HER2 but a part may express circ-HER2 generated from HER2. The generation of this plasmid is a result of chemical gene synthesis of the sequence of exon3-7 of HER2 alongside addition of circulation promoter sequences to 83 bp upstream and 53 bp downstream [42]. Trastuzumab-Deruxtecan has been found to be efficacious in diminishing HER2 positive TNBC. CD3 is another surface marker inhabiting T lymphocytes which has a role of prominence in extinguishing tumor environment [43]. Therefore, simultaneous targeting of both HER2 and CD3 may present remarkable efficacy in eliminating tumor cells. Bispecific antibody like Ertumaxomab addresses this co-targeting necessity by acting on an epitope of HER2 and CD3 by involving Fc fragment to yield active macrophages and antibody dependent cellular cytotoxicity (ADCC) [44]. This achievement demands further investigations and development of similar bispecific antibodies with anti-tumor properties.
Recently, adenosine receptor antagonists are being explored for immunosuppressive myeloid cells in cancer. Two G-protein coupled receptors namely, A2B and predominantly A2A mediate the immunosuppressive action of extracellular adenosine and subsequent blocking of these adenosine receptors enhance anti-tumor immune responses. INCB106385 is a novel drug that has been entrenched as a dual antagonist which binds to both A2A and A2B receptors in the single-digit nanomolar range and antagonizes the production of cAMP in A2A and A2B expressing immune cells [45]. A diagrammatic perspective of this theoretical explanation is depicted in Fig. 2. Further discovery campaigns need to be established for evaluating the efficacy of adenosine receptor antagonists.
Immunosuppressive influence of adenosine binding with A2A and A2B receptors in the tumor micro environment and mechanism of enhancement of anti-tumor response through blocking of these receptors by adenosine receptor antagonists (INCB106385). (adenosine triphosphate (ATP), adenosine monophosphate (AMP), myeloid-derived suppressor cells (MDSC).)
Roadmap of FDA approvals for TNBC
On March 08, 2019, Atezolizumab qualified for an accelerated approval from FDA for its efficacy in unresectable locally advanced or metastatic TNBC with positive PD-L1 expression. Atezolizumab accompanied by nanoparticle albumin-bound (nab)-Paclitaxel received a joint-approval for the formerly mentioned indication based on its prolongation effect on progression-free survival (PFS). Combining nab-Paclitaxel with Atezolizumab improved the anticancer activity [46]. The recommended dose for Atezolizumab is 840 mg administered as an IV infusion over 60 min on days 1 and 15, followed by administration of 100 mg/m2 nab-paclitaxel on days 1, 8 and 15 for each 28-day cycle. This was to be continued until resolution of disease progression or occurrence of any unacceptable toxicity [47]. The FDA assessment of changes in the therapeutic landscape of metastatic TNBC concluded in voluntary withdrawal of accelerated approval of this drug combination by Genentech where drug safety and efficacy parameters were not responsible for withdrawal [48]. The voluntary withdrawal of this accelerated approval of Atezolizumab for this indication accompanied a disappointing phase but continuing extensive research in this field presents a hope of successfully finding an efficacious treatment of TNBC in the near future [49].
Another remarkable drug was added to the armamentarium of TNBC treatment on July 26, 2021, when FDA approved Pembrolizumab for high risk, early-stage TNBC in the capacity of neoadjuvant. Pembrolizumab combined with chemotherapy regimen was found to be efficacious as neoadjuvant chemotherapy and as a sole Pembrolizumab adjuvant after surgery [50]. Combination of Pembrolizumab with chemotherapy had a positive impact on pathological complete response rate (pCR) and event free survival (EFS). The recommended dose of Pembrolizumab is 200 mg every 3 weeks or 400 mg every 6 weeks as an IV infusion over 30 min with neoadjuvant therapy continuing for 24 weeks and adjuvant therapy for 27 weeks [51].
On April 7, 2021, FDA granted approval to Sacituzumab govitecan in patients previously exposed to two or more systemic therapies for unresectable locally advanced or metastatic TNBC [52]. This approval is rooted from its affirmative potential in prolonging PFS and overall survival (OS). Dosage recommendations for Sacituzumab govitecan are 10 mg/kg once a week on days 1 and 8 of 21-day cycle continued until resolution of disease progression or occurrence of any unacceptable toxicity [53]. This timeline of drug development and subsequent FDA approvals is presented diagrammatically in Fig. 3.
Emerging immunotherapies and ongoing investigations have led us to a possibility of more FDA drug approvals for this indication in near future.
Evidence retrieved from clinical trials
Immunotherapy has gained a valuable designation in the interventional sphere of metastatic TNBC and this prompted the investigation of its role in the neoadjuvant setting. Neoadjuvants are a part of an interventional strategy that aim to shrink down the tumor before administration of primary treatment thus improving the efficacy of primary treatment. One trial leaning towards the affirmative potential of Pembrolizumab- a humanized IgG4 monoclonal antibody in combination with chemotherapy as neoadjuvant in TNBC treatment was conducted by enrolling 60 participants and equally dividing the number into six cohorts of Pembrolizumab plus chemotherapy regimens (NCT02622074). Specified primary endpoints were safety and recommended phase II dose (RP2D) and secondary endpoints were pCR rate, objective response rate (ORR), EFS and OS. Endpoints to be explored included defining a relationship between outcome and molecular biomarkers such as PD-L1 expression and stromal tumor-infiltrating lymphocytes. Only two out of total six cohorts met RP2D threshold with 22 patients encountering dose-limited toxicity in the form of febrile neutropenia. The most common grade ≥ 3 treatment-related adverse event was noted as neutropenia (73%). Immune-mediated and infusion reactions were observed in 18 patients with grade ≥ 3 in 6 patients. Across all cohorts, the pCR rate was 60% and 12 month event-free and OS ranged between 80 and 100%. This deduced that administering combination of chemotherapy with Pembrolizumab as neoadjuvant in high-risk, early-stage TNBC showed manageable toxicity and promising antitumor potential [54]. This draws us towards the possibility of administering Pembrolizumab as adjuvant and neoadjuvant therapy in TNBC to potentiate tumor eradication.
Another trial exemplifying role of Atezolizumab- a humanized IgG1 monoclonal antibody combined with Nab-paclitaxel in previously untreated metastatic TNBC was conducted by choosing placebo combined with Nab-paclitaxel as a comparator (NCT02425891) [55]. 902 participants were enrolled with primary end points selected as PFS and OS and secondary outcomes as Objective Response of Complete Response (CR) or Partial Response (PR), Duration of response (DOR), Time to Deterioration (TTD), Percentage of patients with at least one adverse event, percentage of participants with anti-therapeutic antibodies against Atezolizumab, maximum serum concentration of Atezolizumab, minimum serum concentration (Cmin) for Atezolizumab and plasma concentrations of total Paclitaxel. The median PFS was 7.4 months for Atezolizumab plus nab-paclitaxel as compared to 4.8 months for placebo plus nab-paclitaxel receiving patients. ORR and stratified hazard ratio also favoured the administration of combination under study over placebo [46]. This draws a deduction that Atezolizumab plus nab-paclitaxel has a potential to qualify as an interventional strategy in diluting metastatic TNBC.
Although the trials support implication of ICIs in neoadjuvant, adjuvant and metastatic settings of TNBC, these cannot be solely relied upon as a complete cure for this indication as their applicability comes at a cost of incidences of immune-related adverse events (AEs). This demands further investigations to explicate the role of ICI in PD-1/PD-L1 positive TNBC. To summarize, both ongoing and completed clinical trials of immunotherapy are present in Tables 2, 3 and 4 respectively.
Tackling TNBC with vaccines
Under the multitude of immunotherapies, cancer vaccines have been envisioned as a core focus since a long time. Cancer vaccines (CVs) are designed to mount an immune response by recognizing TAAs and destroy cancer cells that residents them [56]. CVs can be categorized into two based on their purpose of usage namely prophylactic and therapeutic CVs. Prophylactic vaccines aim at reducing future incidences while therapeutic CVs are developed to contract existing malignancy [57]. This strategy has also been practiced for development of CVs directed towards breast cancer. The most common targets and TAAs aimed for successful vaccination in BC include E75 peptide, glycoprotein 2 (GP2) peptide, CD4 + lymphocytes, mucin short variant S1 (MUC1) antigen, melanoma associated antigen-3 (MAGE-A3), New York Esophageal Squamous Cell Carcinoma-1 (NY-ESO-1), Carcinoembryogenic antigen (CEA), human telomerase reverse transcriptase (hTERT), Wilms’ tumor antigen (WT-1), NY-BR-1, Mammaglobin-A (Mam-A) and TRICOM. Targets to develop vaccination are summarized in Table 5.
With emerging number of antigens specifically expressed in TNBC, huge scope of vaccine development against this carcinoma is presented. To date, numerous CVs right from peptide-based to DNA, cytokines and lymphocyte-based vaccines are paving their way to qualifying for TNBC treatment [58]. Their progress is hindered by immunologically inadequate clinical settings (large tumor burden in metastatic disease), predominance of TAAs as targets, choice of vaccine delivery platform, concomitant administration of therapies with CV and existence of influential unrestrained mechanisms favouring immune escape including antigen modification, absence of Human Leukocyte Ag class-1 (HLA-1) expression and down-regulation of TAAs which cannot be recognised as a target [59], 60, 61.
Numerous clinical trials are advancing towards the search for a safe and effective vaccine. An open-label, phase 2 study was designed to assess the enhancement in tumor-specific immune response and determine efficacy of Biological AE37 Peptide vaccine combined with Pembrolizumab in metastatic triple-negative breast cancer (mTNBC) patients (NCT04024800). The study progressed with Simon two-step design and from 29 eligible patients, 13 patients were grouped as safety cohort in Stage 1 and were subjected to the combination therapy of AE37 vaccine with Pembrolizumab. The primary outcome measures to be assessed were recommended dose within 72 h of vaccination in first 13 patients and ORR determined by RECIST 1.1. The secondary outcomes selected were PFS, OS, Clinical benefit rate (CBR) and Overall toxicity. The study aims to establish the recommended biologic dose of combined AE37 vaccine with Pembrolizumab and the results of the same are expected soon [62].
Another trial presenting a noteworthy evidence is a phase 1b/2 study that evaluated safety and efficacy of metronomic combination therapy in TNBC patients who progressed on or after standard of care (SoC) chemotherapy (NCT03387085) [63]. Treatment was administered by choosing a 3 week cycle routine in a lower dose (aldoxorubicin, cyclophosphamide, cisplatin, nab-paclitaxel, 5-FU/L), antiangiogenic therapy (bevacizumab), Stereotactic Body Radiation Therapy (SBRT), engineered allogeneic CD16 NK-92 cells (haNK), IL-15RαFc (N-803), adenoviral vector-based CEA, MUC1, brachyury, and HER2 vaccines, yeast vector-based Ras, brachyury and CEA vaccines, and an IgG1 PD-L1 inhibitor, Avelumab. Selected primary endpoints for phase 1b was incidence of treatment-related adverse effect (TRAE) and serious adverse events (SAE). Secondary endpoints assessed were ORR, PFS, OS and Disease control rate (DCR). 8 subjects received 3 treatment cycles in an outpatient setting with all having atleast 1 grade ≥ 3 TRAE being chemotherapy-induced neutropenia. 2 subjects were observed to have grade ≥ 3 haNK- related effects while 2 subjects experienced SAEs. 7 subjects remained alive, 6 subjects continued to receive ongoing treatment while 1 CR and 2 PRs were noted. This trial proved that low dose chemo-radiation combined with innate and adaptive immunotherapy has a good safety profile to be administered in outpatient setting [64]. Clinical trials both ongoing and completed evaluating safety and efficacy of cancer vaccines in TNBC are summarized in Table 6.
Achievable prospects
The edges embodying immunotherapeutic applications in the neoadjuvant, adjuvant and metastatic stages in TNBC have been widened by the preclinical and clinical evidences. Adding to this, cumulative facts obtained from other solid tumors suggest early biopsy to hold a promising potential in revealing the extent of immunotherapeutic benefits reaching to the patient. Unveiling the entire spectrum of clinical benefits of immunotherapy is possible by development of specific biomarkers to precisely predict the response and possible resistance to given immunotherapy. A rational clinical trial design assisted with strong sample collection approach could provide more reliability and accuracy to the efficacy of immunotherapy in diminishing TME. A comprehensive understanding of the fundamentals of TNBC heterogeneity, molecular biomarkers, immunotherapy mechanism cascades and development of resistance could facilitate development of better immunotherapeutic regimens with maximized benefits. Novel approaches to overcome the obstacle of narrow therapeutic index of these drugs should be developed. Identification of clearly defined indications of these drugs as monotherapy and in combination is necessary to achieve increment in drug prescription rationality.
Conclusion
Extending the therapeutic strategies to include immunotherapy in eradication of TNBC has become a necessity due to the shortcomings of conventional approaches and poor prognosis of this subtype of BC. Clinical trials included lean towards the successful use of immunotherapy in neoadjuvant, adjuvant and metastatic TNBC but this is accompanied with occurrences of alarming AEs which can be perceived as a major setback of immunotherapy. Despite the possibility of unfavourable AEs, immunotherapy still can be envisioned as a potential strategy to target tumor cells as benefits outweigh the risks. Clinical trials contribute encouraging results in favour of applicability of vaccines and concrete evidence regarding the same may be expected soon. With ongoing investigations, newly established immunotherapies may prove to qualify as first line therapies for primary TNBC diagnoses. It may also enhance survival parameters in patients presenting with metastasis or recurrence.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- BC:
-
Breast Cancer
- BL1/BL2:
-
Basal-like 1/Basal-like 2
- CAR-T:
-
Chimeric antigen receptor T-cell therapy
- CBR:
-
Clinical benefit rate
- CEA:
-
Carcinoembryogenic antigen
- circ-HER2:
-
Circular human-epidermal growth factor
- CR:
-
Complete response
- CTLA-4:
-
Cytotoxic T lymphocyte-associated antigen-4
- CVs:
-
Cancer vaccines
- DC:
-
Dendritic cells
- DCR:
-
Disease control rate
- DOR:
-
Duration of response
- EFS:
-
Event-free survival
- EGFR:
-
Epidermal growth factor receptor
- ER:
-
Estrogen receptor
- ERα+:
-
Estrogen receptor a-positive
- GP2:
-
Glycoprotein 2
- HER2:
-
Human-epidermal growth factor
- hTERT:
-
Human telomerase reverse transcriptase
- ICIs:
-
Immune checkpoint inhibitors
- IDO:
-
Indoleamine-2, 3-dioxygenase
- LAG-3:
-
Lymphocyte activation gene-3
- LAR:
-
Luminal androgen receptor
- MAGE-A3:
-
Melanoma associated antigen-3
- Mam-A:
-
Mammaglobin-A
- MSLN:
-
Mesothelin
- mTNBC:
-
Metastatic triple-negative breast cancer
- MUC1:
-
Mucin short variant S1
- NY-ESO-1:
-
New York Esophageal Squamous Cell Carcinoma-1
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- pCR:
-
Pathological complete response
- PD-1/PD-L1:
-
Programmed death-1 receptor-ligand-1
- PD-L1:
-
Programmed death ligand 1
- PFS:
-
Progression-free survival
- PK:
-
Pharmacokinetics
- PR:
-
Partial Response
- PR:
-
Progesterone receptor
- PR+:
-
Progesterone receptor-positive
- RP2D:
-
Recommended phase II dose
- SAE:
-
Serious adverse events
- STAT3:
-
Signal transducer and activator of transcription 3
- TAAs:
-
Tumor-associated antigens
- TME:
-
Tumor microenvironment
- TNBC:
-
Triple-negative breast cancer
- TRAE:
-
Treatment-related adverse effect
- TSAs:
-
Tumor specific antigens
- TTD:
-
Time to Deterioration
- VEGF:
-
Vascular endothelial growth factor
- WT-1:
-
Wilms’ tumor antigen
References
Khadela A, Bhikadiya V, Vyas B. Impact of oncology pharmacist services on humanistic outcome in patients with breast cancer. J Oncol Pharm Pract. 2021;28(2):302–9. https://doi.org/10.1177/1078155220988333.
“In Developed and Developing Countries, Breast Cancer Risk is Reduced by 4% for Each Year of Breastfeeding | Guttmacher Institute.” https://www.guttmacher.org/journals/psrh/2002/11/developed-and-developing-countries-breast-cancer-risk-reduced-4-each-year. Accessed 26 Nov 2022
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer—epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—an updated review. Cancers (Basel). 2021;13:17. https://doi.org/10.3390/CANCERS13174287.
Khadela A, Joshi S, Vyas B, Lodha S, Bambharoliya T. “Assessment of prescribing pattern of anti-cancer agents in breast cancer patients at West Indian oncology hospital. Acta Sci cancer biol. 2020. https://doi.org/10.31080/ASCB.2020.04.0247.
Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (London). 2019;11:151. https://doi.org/10.2147/BCTT.S176070.
Ginsburg O, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet (London, England). 2017;389(10071):847–60. https://doi.org/10.1016/S0140-6736(16)31392-7.
Tomao F, et al. Triple-negative breast cancer: new perspectives for targeted therapies. Onco Targets Ther. 2015;8:177–93. https://doi.org/10.2147/OTT.S67673.
Kutomi G, et al. Current status of the prognostic molecular biomarkers in breast cancer: a systematic review. Oncol Lett. 2017;13(3):1491–8. https://doi.org/10.3892/OL.2017.5609/HTML.
Tang Y, Wang Y, Kiani MF, Wang B. Classification, Treatment strategy, and associated drug resistance in breast cancer. Clin Breast Cancer. 2016;16(5):335–43. https://doi.org/10.1016/J.CLBC.2016.05.012.
Feng Y, et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5(2):77–106. https://doi.org/10.1016/J.GENDIS.2018.05.001.
Won KA, Spruck C. Triple-negative breast cancer therapy: current and future perspectives (Review). Int J Oncol. 2020;57(6):1245. https://doi.org/10.3892/IJO.2020.5135.
Derakhshan F, Reis-Filho JS. Pathogenesis of triple-negative breast cancer. Annu Rev Pathol. 2022;17:181. https://doi.org/10.1146/ANNUREV-PATHOL-042420-093238.
Sukumar J, Gast K, Quiroga D, Lustberg M, Williams N. Triple-negative breast cancer: promising prognostic biomarkers currently in development. Expert Rev Anticancer Ther. 2021;21(2):135. https://doi.org/10.1080/14737140.2021.1840984.
Medina MA, et al. Triple-negative breast cancer: a review of conventional and advanced therapeutic strategies. Int J Environ Res Public Health. 2020;17:6. https://doi.org/10.3390/IJERPH17062078.
Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–21. https://doi.org/10.1038/s41423-020-0488-6.
Bai X, Ni J, Beretov J, Graham P, Li Y. Immunotherapy for triple-negative breast cancer: a molecular insight into the microenvironment, treatment, and resistance. J Natl Cancer Cent. 2021;1(3):75–87. https://doi.org/10.1016/J.JNCC.2021.06.001.
Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29:241–50. https://doi.org/10.1016/J.BREAST.2016.07.015.
Thomas R, Al-Khadairi G, Decock J. Immune checkpoint inhibitors in triple negative breast cancer treatment: promising future prospects. Front Oncol. 2021;10:3464. https://doi.org/10.3389/FONC.2020.600573/BIBTEX.
Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interf Cytokine Res. 2015;35(1):1. https://doi.org/10.1089/JIR.2014.0026.
Yang YH, Liu JW, Lu C, Wei JF. CAR-T cell therapy for breast cancer: from basic research to clinical application. Int J Biol Sci. 2022;18(6):2609. https://doi.org/10.7150/IJBS.70120.
McCafferty MPJ, Healy NA, Kerin MJ. Breast cancer subtypes and molecular biomarkers. Diagnostic Histopathol. 2009;15(10):485–9. https://doi.org/10.1016/J.MPDHP.2009.07.002.
Del Bano J, et al. A bispecific antibody-based approach for targeting mesothelin in triple negative breast cancer. Front Immunol. 2019;10:1593. https://doi.org/10.3389/FIMMU.2019.01593/BIBTEX.
Lv J, Li P. Mesothelin as a biomarker for targeted therapy. Biomark Res. 2019;7(1):1–18. https://doi.org/10.1186/S40364-019-0169-8.
Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7(1):18. https://doi.org/10.1186/S40169-018-0198-1.
Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234(6):8381–95. https://doi.org/10.1002/JCP.27740.
Tume L, Paco K, Ubidia-Incio R, Moya J. CD133 in breast cancer cells and in breast cancer stem cells as another target for immunotherapy. Gac Mex Oncol. 2016;15(1):22–30. https://doi.org/10.1016/J.GAMO.2016.01.003.
Ghahremani Dehbokri S, et al. CTLA-4: As an Immunosuppressive Immune Checkpoint in Breast Cancer. Curr Mol Med. 2022. https://doi.org/10.2174/1566524022666220610094716.
Chen X, et al. CTLA-4 positive breast cancer cells suppress dendritic cells maturation and function. Oncotarget. 2017;8(8):13703–15. https://doi.org/10.18632/ONCOTARGET.14626.
Navarrete-Bernal MGC, et al. Biological landscape of triple negative breast cancers expressing CTLA-4. Front Oncol. 2020;10:1206. https://doi.org/10.3389/FONC.2020.01206/BIBTEX.
He M, et al. Remarkably similar CTLA-4 binding properties of therapeutic ipilimumab and tremelimumab antibodies. Oncotarget. 2017;8(40):67129. https://doi.org/10.18632/ONCOTARGET.18004.
Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727.
Zhu H, et al. PD-1/PD-L1 counterattack alliance: multiple strategies for treating triple-negative breast cancer. Drug Discov Today. 2020;25(9):1762–71. https://doi.org/10.1016/J.DRUDIS.2020.07.006.
Lopez-Beltran A, et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers. 2021;13(1):131. https://doi.org/10.3390/CANCERS13010131.
Shi AP, et al. Immune checkpoint LAG3 and its ligand FGL1 in cancer. Front Immunol. 2022. https://doi.org/10.3389/FIMMU.2021.785091/FULL.
Wang Y, Dong T, Xuan Q, Zhao H, Qin L, Zhang Q. Lymphocyte-activation gene-3 expression and prognostic value in neoadjuvant-treated triple-negative breast cancer. J Breast Cancer. 2018;21(2):124–33. https://doi.org/10.4048/JBC.2018.21.2.124.
Stovgaard ES, et al. Prognostic and clinicopathologic associations of LAG-3 expression in triple-negative breast cancer. Appl Immunohistochem Mol Morphol. 2021. https://doi.org/10.1097/PAI.0000000000000954.
Nilsson L, et al. Patient characteristics influence activated signal transducer and activator of transcription 3 (STAT3) levels in primary breast cancer—impact on prognosis. Front Oncol. 2020;10:1278. https://doi.org/10.3389/FONC.2020.01278/BIBTEX.
Ma JH, Qin L, Li X. Role of STAT3 signaling pathway in breast cancer. Cell Commun Signal. 2020;18(1):1–13. https://doi.org/10.1186/S12964-020-0527-Z/FIGURES/5.
Wei L, et al. High indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front Immunol. 2018;9:724. https://doi.org/10.3389/FIMMU.2018.00724/BIBTEX.
Asghar K, et al. Indoleamine 2,3-dioxygenase expression and overall survival in patients diagnosed with breast cancer in Pakistan. Cancer Manag Res. 2019;11:475. https://doi.org/10.2147/CMAR.S184221.
Godfrey JD, et al. HER2 c-terminal fragments are expressed via internal translation of the HER2 mRNA. Int J Mol Sci. 2022;23(17):9549. https://doi.org/10.3390/IJMS23179549.
Li J, et al. Circular HER2 RNA positive triple negative breast cancer is sensitive to Pertuzumab. Mol Cancer. 2020;19:1. https://doi.org/10.1186/S12943-020-01259-6.
Yu S, et al. A novel asymmetrical anti-HER2/CD3 bispecific antibody exhibits potent cytotoxicity for HER2-positive tumor cells. J Exp Clin Cancer Res. 2019;38(1):1–16. https://doi.org/10.1186/S13046-019-1354-1/FIGURES/7.
Dillon PM, Tushir-Singh J, Lum LG. Bispecific antibodies for the treatment of breast cancer. Expert Opin Biol Ther. 2021;22(8):1017–27. https://doi.org/10.1080/14712598.2021.1922665.
Wang H, et al. Abstract LB157: Discovery and characterization of INCB106385: a novel A2A/A2B adenosine receptor antagonist, as a cancer immunotherapy. Cancer Res. 2021;81(13):LB157–LB157. https://doi.org/10.1158/1538-7445.AM2021-LB157.
Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. https://doi.org/10.1056/NEJMOA1809615/SUPPL_FILE/NEJMOA1809615_DATA-SHARING.PDF.
Emens LA, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol. 2021;32(8):983–93. https://doi.org/10.1016/j.annonc.2021.05.355.
“Genentech withdraws accelerated approval of atezolizumab regimen for breast cancer subset.” https://www.healio.com/news/hematology-oncology/20210827/genentech-withdraws-accelerated-approval-of-atezolizumab-regimen-for-breast-cancer-subset. Accessed 26 Nov 2022
Wojtukiewicz MZ, Pogorzelska M, Politynska B. Immunotherapy for triple negative breast cancer: the end of the beginning or the beginning of the end? Cancer Metastasis Rev. 2022;41(3):465–9. https://doi.org/10.1007/S10555-022-10060-4/FIGURES/1.
Schmid P, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–67. https://doi.org/10.1056/NEJMOA2112651/SUPPL_FILE/NEJMOA2112651_DATA-SHARING.PDF.
Schmid P, et al. Pembrolizumab for early triple-Negative breast cancer. N Engl J Med. 2020;382(9):810–21. https://doi.org/10.1056/NEJMOA1910549/SUPPL_FILE/NEJMOA1910549_DATA-SHARING.PDF.
Bardia A, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–41. https://doi.org/10.1056/NEJMOA2028485/SUPPL_FILE/NEJMOA2028485_DATA-SHARING.PDF.
Olivier T, Prasad V. Sacituzumab govitecan in metastatic triple negative breast cancer (TNBC): four design features in the ASCENT trial potentially favored the experimental arm. Transl Oncol. 2022;15:1. https://doi.org/10.1016/J.TRANON.2021.101248.
Schmid P, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31(5):569–81. https://doi.org/10.1016/J.ANNONC.2020.01.072.
“A Study of Atezolizumab in Combination With Nab-Paclitaxel Compared With Placebo With Nab-Paclitaxel for Participants With Previously Untreated Metastatic Triple-Negative Breast Cancer (IMpassion130) - Full Text View–ClinicalTrials.gov.” https://clinicaltrials.gov/ct2/show/NCT02425891. Accessed 26 Nov 2022
Shemesh CS, et al. Personalized cancer vaccines: clinical landscape, challenges, and opportunities. Mol Ther. 2021;29(2):555–70. https://doi.org/10.1016/J.YMTHE.2020.09.038.
DeMaria PJ, Bilusic M. Cancer Vaccines. Hematol Oncol Clin North Am. 2019;33(2):199–214. https://doi.org/10.1016/j.hoc.2018.12.001.
Li Z, Qiu Y, Lu W, Jiang Y, Wang J. Immunotherapeutic interventions of triple negative breast cancer. J Transl Med. 2018;16(1):1–19. https://doi.org/10.1186/S12967-018-1514-7.
Corti C, Giachetti PPMB, Eggermont AMM, Delaloge S, Curigliano G. Therapeutic vaccines for breast cancer: has the time finally come? Eur J Cancer. 2022;160:150–74. https://doi.org/10.1016/J.EJCA.2021.10.027.
Antonarelli G, et al. Therapeutic cancer vaccines revamping: technology advancements and pitfalls. Ann Oncol. 2021;32(12):1537. https://doi.org/10.1016/J.ANNONC.2021.08.2153.
Rini BI, et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(11):1599–611. https://doi.org/10.1016/S1470-2045(16)30408-9.
“Establishing the Recommended Biological Dose for AE37 Peptide Vaccine in Combination With Pembrolizumab That Will Enhance the Tumor-specific Immune Response and Demonstrate Efficacy in Patients With Advanced Triple-negative Breast Cancer—Full Text View—ClinicalTrials.gov.” https://clinicaltrials.gov/ct2/show/NCT04024800. Accessed 22 Sep 2022
“QUILT-3.067: NANT triple negative breast cancer (tnbc) vaccine: molecularly informed integrated immunotherapy in subjects with tnbc who have progressed on or after standard-of-care therapy—full text view—clinicaltrials.gov.” https://www.clinicaltrials.gov/ct2/show/NCT03387085. Accessed 22 Sep 2022
Nangia CS, Kistler M, Sender LS, Lee JH, Jones FR, Jafari O, et al. Innate and adaptive immunotherapy: an orchestration of immunogenic cell death by overcoming immune suppression and activating NK and T-cell therapy in patients with third line or greater TNBC. J Clin Oncol. 2019;37:e12566. https://doi.org/10.1200/JCO.2019.37.15_SUPPL.E12566
“Basket trial for combination therapy with durvalumab (Anti-PDL1) (MEDI4736) and tremelimumab (Anti-CTLA4) in patients with metastatic solid tumors—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03982173. Accessed 30 Nov 2022
“Evaluate the clinical benefit of a post-operative treatment associating Radiotherapy + nivolumab + ipilimumab versus radiotherapy + capecitabine for triple negative breast cancer patients with residual disease—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03818685. Accessed 30 Nov 2022
“LN-145 or LN-145-S1 in treating patients with relapsed or refractory ovarian cancer, triple negative breast cancer (TNBC), anaplastic thyroid cancer, osteosarcoma, or other bone and soft tissue sarcomas—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03449108. Accessed 30 Nov 2022
“OXEL: immune checkpoint or capecitabine or combination therapy as adjuvant therapy for tnbc with residual disease—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03487666. Accessed 30 Nov 2022
“Immune induction strategies to improve response to immune checkpoint blockade in triple negative breast cancer (TNBC) patients—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT04159818. Accessed 30 Nov 2022
“A study of atezolizumab plus nab-paclitaxel in the treatment of unresectable locally advanced or metastatic PD-L1-positive triple-negative breast cancer—Full Text View—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT04148911. Accessed 30 Nov 2022
“Clinical trial of neoadjuvant chemotherapy with atezolizumab or placebo in patients with triple-negative breast cancer followed after surgery by atezolizumab or placebo—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03281954. Accessed 30 Nov 2022
Pérez-García J, Soberino J, Racca F, Gion M, Stradella A, Cortés J. Atezolizumab in the treatment of metastatic triple-negative breast cancer. Expert Opin Biol Ther. 2020;20(9):981–9. https://doi.org/10.1080/14712598.2020.1769063.
“Atezolizumab combined with immunogenic chemotherapy in patients with metastatic triple-negative breast cancer—tabular view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT03164993?term=PD1%2FPDL1%2C+Atezolizumab&cond=Breast+Cancer&draw=2&rank=2. Accessed 03 Oct 2022
“A study comparing atezolizumab (anti PD-L1 antibody) in combination with adjuvant anthracycline/taxane-based chemotherapy versus chemotherapy alone in patients with operable triple-negative breast cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03498716. Accessed 30 Nov 2022
“Investigate the contribution of ipatasertib to neoadjuvant chemotherapy plus atezolizumab in TNBC—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT05498896. Accessed 30 Nov 2022
“Evaluating the efficacy and safety of bevacizumab, carboplatin, gemcitabine and atezolizumab in breast cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT04739670. Accessed 30 Nov 2022
“A study of the efficacy and safety of atezolizumab plus chemotherapy for patients with early relapsing recurrent triple-negative breast cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03371017. Accessed 30 Nov 2022
“Study to compare a mono atezolizumab window followed by a atezolizumab - ctx therapy with atezolizumab—ctx therapy—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT04770272?term=NCT04770272&draw=2&rank=1. Accessed 03 Oct 2022
“Efficacy and safety of atezolizumab plus capecitabine adjuvant therapy for triple receptor-negative breast cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03756298. Accessed 30 Nov 2022
“Nab-paclitaxel and atezolizumab before surgery in treating patients with triple negative breast cancer—tabular view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT02530489?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=30. Accessed 03 Oct 2022
“First Line atezolizumab, paclitaxel, and bevacizumab (Avastin®) in mTNBC—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT04408118. Accessed 30 Nov 2022
“Carboplatin and paclitaxel with or without atezolizumab before surgery in treating patients with newly diagnosed, stage ii–iii triple-negative breast cancer—tabular view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT02883062?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2. Accessed 03 Oct 2022
“Atezolizumab + sacituzumab govitecan to prevent recurrence in tnbc (aspria)—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT04434040. Accessed 11 Oct 2022
“Atezolizumab plus carboplatin plus nab-paclitaxel—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT05266937. Accessed 30 Nov 2022
“A clinical study of TJ004309 with Atezolizumab (TECENTRIQ®) in patients with ovarian cancer and selected solid tumors—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT05001347. Accessed 30 Nov 2022
“Adjuvant treatment for high-risk triple negative breast cancer patients with the anti-PD-l1 antibody avelumab—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT02926196. Accessed 30 Nov 2022
“Restoring sensitivity to immunotherapy in advanced triple negative breast cancer exploiting ceralasertib priming followed by combined durvalumab/nab-paclitaxel—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT05582538. Accessed 30 Nov 2022
“Radiation, immunotherapy and parp inhibitor in triple negative breast cancer - tabular view - clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT04837209?term=immunotherapy&recrs=abdf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=5. Accessed 03 Oct 2022
“Reinvigorating TNBC response to immunotherapy with combination myeloid inhibition and radiation—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT05491226. Accessed 30 Nov 2022
“Dendritic cell-based treatment plus immunotherapy for the treatment of metastatic or unresectable triple negative breast cancer—full text view— clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT05539365. Accessed 30 Nov 2022
“Panitumumab and pembrolizumab in combination with neoadjuvant chemotherapy for the treatment of stage III–IV triple negative breast cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT05177796?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=15. Accessed 03 Oct 2022
“Reverse triple negative immune resistant breast cancer—tabular view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT05076682?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2. Accessed 03 Oct 2022
“Neoadjuvant phase II study of pembrolizumab and carboplatin plus docetaxel in triple negative breast cancer—tabular view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT03639948?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=20. Accessed 03 Oct 2022
“Pre-op pembro + radiation therapy in breast cancer (P-RAD)—tabular view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT04443348?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=23. Accessed 03 Oct 2022
“Pembrolizumab in treating patients with stage iv metastatic or recurrent inflammatory breast cancer or triple-negative breast cancer who have achieved clinical response or stable disease to prior chemotherapy—tabular view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT02411656?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=35. Accessed 03 Oct 2022
“The efficacy and safety of sintilimab plus anlotinib combined with chemotherapy as neoadjuvant therapy in TNBC—tabular view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT04877821?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=38. Accessed 03 Oct 2022
“Neoadjuvant Pembrolizumab + decitabine followed by std neoadj chemo for locally advanced HER2- breast Ca—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/study/NCT02957968?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=4&rank=50. Accessed 03 Oct 2022
“Efficacy of spartalizumab across multiple cancer-types in patients with PD1-high mRNA expressing tumors—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT04802876?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=54. Accessed 03 Oct 2022
“Testing MK-3475 (Pembrolizumab) as adjuvant therapy for triple receptor-negative breast cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT02954874?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=55. Accessed 03 Oct 2022
“RAPA-201 therapy of solid tumors—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT05144698?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=62. Accessed 03 Oct 2022
“Safety and efficacy of KY1044 and atezolizumab in advanced cancer—full text view–clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03829501. Accessed 30 Nov 2022
“Study of safety and efficacy of novel immunotherapy combinations in patients with triple negative breast cancer (TNBC). - Tabular View - ClinicalTrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT03742349?term=immunotherapy&recrs=abdf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=2. Accessed 03 Oct 2022
“Neoadjuvant MEDI4736 concomitant with weekly nab-paclitaxel and dose-dense AC for stage I–III triple negative breast cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT02489448. Accessed 30 Nov 2022
Winer EP, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(4):499–511. https://doi.org/10.1016/S1470-2045(20)30754-3.
“Study of imprime PGG and pembrolizumab in advanced melanoma and triple negative breast cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT02981303. Accessed 30 Nov 2022
“Study to assess the efficacy of pembrolizumab plus radiotherapy in metastatic triple negative breast cancer patients—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT02730130. Accessed 30 Nov 2022
Adams S, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(3):397–404. https://doi.org/10.1093/ANNONC/MDY517.
“Niraparib in combination with pembrolizumab in patients with triple-negative breast cancer or ovarian cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT02657889. Accessed 30 Nov 2022
“A study to investigate atezolizumab and chemotherapy compared with placebo and chemotherapy in the neoadjuvant setting in participants with early stage triple negative breast cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03197935. Accessed 30 Nov 2022
“A study to test the safety and effectiveness of nivolumab combined with daratumumab in patients with pancreatic, non-small cell lung or triple negative breast cancers, that have advanced or have spread—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03098550?term=NCT03098550&draw=2&rank=1. Accessed 03 Oct 2022
“A phase II study of nivolumab in combination with cabozantinib for metastatic triple-negative breast cancer—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03316586. Accessed 30 Nov2022
“Study to explore the safety, tolerability and efficacy of mk-3475 in combination with INCB024360 in participants with selected cancers—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT02178722. Accessed 30 Nov 2022
Powderly J, et al. Phase I evaluation of AB928, a novel dual adenosine receptor antagonist, combined with chemotherapy or AB122 (anti-PD-1) in patients (pts) with advanced malignancies. Ann Oncol. 2019;30: v493. https://doi.org/10.1093/annonc/mdz253.032.
Labrie M, et al. Multiomics analysis of serial PARP inhibitor treated metastatic TNBC inform on rational combination therapies. NPJ Precis Oncol. 2021;5:1. https://doi.org/10.1038/S41698-021-00232-W.
“Study of talimogene laherparepvec with atezolizumab for triple negative breast cancer and colorectal cancer with liver metastases—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT03256344?term=NCT03256344&draw=2&rank=1. Accessed 03 Oct 2022
Schmid P, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(5):569–81. https://doi.org/10.1016/J.ANNONC.2020.01.072.
Schneeweiss A, et al. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto-GBG 84): a randomised phase III trial. Eur J Cancer. 2019;106:181–92. https://doi.org/10.1016/J.EJCA.2018.10.015.
O’Shaughnessy J, Roberts LK, Smith JL, Levin MK, Timis R, Finholt JP, et al. Safety and initial clinical efficacy of a dendritic cell (DC) vaccine in locally advanced, triple-negative breast cancer (TNBC) patients (pts). J Clin Oncol. 2016;34:1086. https://doi.org/10.1200/JCO.2016.34.15_SUPPL.1086
Bernal-Estévez D, Sánchez R, Tejada RE, Parra-López C. Chemotherapy and radiation therapy elicits tumor specific T cell responses in a breast cancer patient. BMC Cancer. 2016;16:1. https://doi.org/10.1186/S12885-016-2625-2.
“Breast cancer neoantigen vaccination with autologous dendritic cells—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT04105582?term=immunotherapy&recrs=e&cond=Triple+Negative+Breast+Cancer&draw=1&rank=2. Accessed 03 Oct 2022
“PVX-410 vaccine plus pembrolizumab in HLA-A2+ metastatic triple negative breast cancer—tabular view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT03362060?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=12. Accessed 03 Oct 2022
“Adjuvant PVX-410 vaccine and durvalumab in stage II/III triple negative breast cancer—tabular view—clinicalTrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT02826434?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=18. Accessed 03 Oct 2022
“Testing the addition of an individualized vaccine to nab-paclitaxel, durvalumab and tremelimumab and chemotherapy in patients with metastatic triple negative breast cancer—tabular view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/record/NCT03606967?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=29. Accessed 03 2022
“Personalized neoantigen peptide-based vaccine in combination with pembrolizumab for the treatment of advanced solid tumors, the pneovca study—full text view—clinicaltrials.gov.” https://clinicaltrials.gov/ct2/show/NCT05269381?term=immunotherapy&recrs=adf&cond=Triple+Negative+Breast+Cancer&draw=2&rank=65. Accessed 03 Oct 2022
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Study conception, design, manuscript draft review and editing were performed by AK, SS and ACB. The first draft of the manuscript was written by ACS, AJP, and KM. The data curation was performed by NRK and IS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khadela, A., Soni, S., Megha, K. et al. Contracting triple-negative breast cancer with immunotherapeutic armamentarium: recent advances and clinical prospects. Med Oncol 40, 48 (2023). https://doi.org/10.1007/s12032-022-01922-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01922-6