Abstract
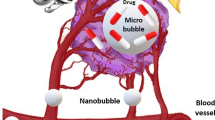
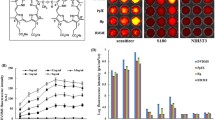
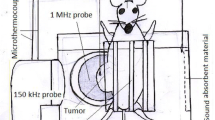
This study aimed to evaluate the effect of dual-frequency sonication in the presence of thalidomide angiogenesis inhibitor and nanomicelles containing doxorubicin on inhibiting the growth and angiogenesis of breast adenocarcinoma in BALB/c female mice. Sixty mice carrying the tumor were divided into 12 groups: (A) control, (B) 28 kHz and 3 MHz sonication, (C) thalidomide, (D) thalidomide and 28 kHz, (E) thalidomide and 3 MHz, (F) thalidomide and dual-frequency sonication, (G) doxorubicin, (H) nanomicelles containing doxorubicin, (I) nanomicelles containing doxorubicin and dual-frequency sonication, (J) thalidomide and doxorubicin, (K) thalidomide and nanomicelles containing doxorubicin, and (L) thalidomide and nanomicelles containing doxorubicin and dual-frequency sonication. The delay in the tumor growth and angiogenesis percent were extracted. Pathological and immunohistochemical studies were performed to confirm the treatment. The findings of tumor growth retardation parameters and animal survival were significantly different in group L from all groups (P < 0.05). The highest rate of inhibition was in group L with a 46% inhibition. In group L, 100% of the animals survived until day 49. In groups F, C, G, B, and A, all the animals survived 45, 42, 39, 32, and 30 days, respectively. Pathological results showed a decrease in tumor grade in groups K and L. Histopathological results demonstrate a decrease in group L angiogenesis compared to group C. These findings were consistent with the results of color Doppler ultrasound imaging. Dual-frequency sonication in the presence of thalidomide and doxorubicin-containing nanomicelles inhibits tumor growth and angiogenesis.








Similar content being viewed by others
Data availability
All relevant data are included within the paper and supporting information files. Please contact the corresponding author for material availability.
References
Roberta L, Mohanraj R, Anna D. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1754–70.
Kadosawa T, Watabe A. The effects of surgery-induced immunosuppression and angiogenesison tumor growth. Vet J. 2015;205:175–9.
Lalita Y, Naveen P, Varun R, Pranali S, Vandana SH. Tumour angiogenesis and angiogenic inhibitors: a review. J Clin Diagn Res. 2015;9:1–5.
Kim SW, Park SS, Ahn SJ, Chung KW, Moon WK, Im JG, Yeo JS, Chung JK, Noh DY. Identification of angiogenesis in primary breast carcinoma according to the image analysis. Breast Cancer Res Treat. 2002;74:121–9.
Chong S, Jianmin L, Bin W, Janjie S, Matteo F, Na S, Liang P, Zhuoli Z. Tumor angiogenesis and bone metastasis-Correlation in invasive breast carcinoma. J Immunol Methods. 2018;452:46–52.
Deshpande N, Pysz MA, Willmann JK. Molecular ultrasound assessment of tumor angiogenesis. Angiogenesis. 2010;13:175–88.
Mercurio A, Adriani G, Catalano A, Carocci A, Rao L. A mini-review on thalidomide: chemisty, mechanisms of action, therapeutic potential and anti-angiogenic properties in multiple myeloma. Curr Med Chem. 2017;24:2736–44.
Chung F, Lu J, Palmer BD, Kestell P, Browett P, Baguley BC, Tingle M, Ching LM. Thalidomide pharmacokinetics and metabolite formation in mice, rabbits, and multiple myeloma patients. Clin Cancer Res. 2004;10:5949–56.
Siegel R, Miller K, Jemal A. Cancer statistics, 2020. Cancer J Clin. 2020;70:7–30.
Wood AK, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med Biol. 2015;41:905–28.
Priyanka T, Ramaya V, Wu S, Craig J, Wojci C. Nano-enhanced drug delivery and therapeutic ultrasound for cancer treatment and beyond. Front Bioeng Biotechnol. 2019;7:1–26.
Dingjie S, Bala G, Shengqi Z, Yun J. Numerical investigation of the inertial cavitation threshold under multi-frequency ultrasound. Ultrson Sonochem. 2018;4:419–26.
Ciuti P, Dezhkunov NV, Francescutto A, Calligaris F, Sturman F. Study into mechanisms of the enhancement of multibubble sonoluminescence emission in interacting fields of different frequencies. Ultrason Sonochem. 2003;10:337–41.
Baghbani F, Chegeni M, Moztarzadeh F, Aghazadeh J, Mokhtari-Dizaji M. Ultrasonic nanotherapy of breast cancer using novel ultrasound responsive alginate shelled perfluorohexane nanodroplets: in vitro and in vivo evaluation. Mat Sci Eng C. 2017;77:698–707.
Hasanzadeh H, Mokhtari-Dizaji M, Bathaie Z, Hassan ZM. Effect of local dual frequency sonication on drug distribution from polymeric nanomicelleles. Ultrason Sonochem. 2011;18:1165–71.
Tanbour R, Martin A, Pitt W, Husseini G. Drug delivery systems based on polymeric micelles and ultrasound: a review. Curr Pharm Des. 2016;22:2796–807.
Baghbani F, Moztarzadeh F, Mohandesi JA, Yazdian F, Mokhtari-Dizaji M. Novel alginate-stabilized doxorubicin-loaded nanodroplets for ultrasonic theranosis of breast cancer. Int J Biol Macromol. 2016;93:512–9.
Hasanzadeh H, Mokhtari-Dizaji M, Bathaie Z, Hassan ZM, Nilchiani V, Goudarzi H. Enhancement and control of acoustic cavitation yield by low-level dual frequency sonication: a subharmonic analysis. Ultrason Sonochem. 2011;18:394–400.
Emoto M, Tachibana K, Iwasaki H, Kawarabayashi T. Antitumor effect of TNP-470, an angiogenesis inhibitor, combined with ultrasound irradiation for human uterine sarcoma xenografts evaluated using contrast color Doppler ultrasound. Cancer Sci. 2007;98:929–35.
Zhang C, Huang P, Zhang Y, Chen J, Shentu W, Sun Y, Yang Z, Chen S. Anti-tumor efficacy of ultrasonic cavitation is potentiated by concurrent delivery of anti-angiogenic drug in colon cancer. Cancer Lett. 2014;347:105–13.
Yao M, Jiaxuan H, Jiaxuan J, Zhiwei Z, Yandi T, Chaoqi L, Yun Z. Ultrasound targeting of microbubble- bound anti PD-LT mAb to enhance anti-tumor effect of cisplatin in cervical cancer xenografts treatment. Life Sci. 2020;262:1165–85.
Gijung K, Sung D, Dongkyu K, Hyosuk K, Myung G, Kwangmey K, Lck C, Sun H. Synergistic antitumor effects of combination treatment with metronomic doxorubicin and VEGF-targeting RNAi nanomarticles. J Control Release. 2017;267:203–13.
Souza CM, Carvalho LF, Vieira TS, Silva CA, Miriam Teresa PL, Ferreira MAND, Andrade SP, Cassali GD. Thalidomide attenuates mammary cancer associated-inflammation, angiogenesis and tumor growth in mice. Biomed Pharmacother. 2012;66:491–8.
Ebrahiminia A, Mokhtari-Dizaji M, Toliyat T. Dual frequency cavitation event sensor with iodide dosimeter. Ultrason Sonochem. 2016;28:276–82.
Eggen S, Fagerland S, Hansen R, Berg S, Furu H, Lilledahl M, Angelsen A. Ultrasound-enhanced drug delivery in prostate cancer xenografts by nanoparticles stabilizing microbubbles. J Control Release. 2014;178:39–49.
Vasvari GP, Dychhoff G, Kashfi F, Lemke B, Lohr J, Helmke BM, Schirrmacher V, Plinkert PK, Bechhore P, Herold-Mende CC. Combination of thalidomide and cisplatin in an head and neck squamous cell carcinomas model results in an enhanced antiangiogenic activity in vitro and in vivo. Cancer. 2007;121:1697–704.
Acknowledgements
This study was approved by the Faculty of Medical Sciences, Tarbiat Modares University.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. MMD, as the corresponding author, was the chief investigator. ZG and MMD participated in the design of the work and data and image acquisition; they conducted the statistical analysis, drafted the manuscript, and reviewed and revised this paper. TT participated in data acquisition and design and preparation of nanomicelles. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
NonE.
Ethical approval
The animal experiments followed the guidelines of the Laboratory Animal Ethical Committee of the Faculty of Medical Sciences, Tarbiat Modares University (IR.TMU.REC.1396.33).
Consent for publication
This article has been read and approved in the present form for submission by all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goleh, Z., Mokhtari-Dizaji, M. & Toliyat, T. The effect of dual-frequency sonication in the presence of thalidomide angiogenesis inhibitor and nanomicelles containing doxorubicin on inhibiting the growth and angiogenesis of breast adenocarcinoma in vivo. Med Oncol 40, 20 (2023). https://doi.org/10.1007/s12032-022-01898-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01898-3