Abstract
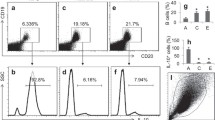
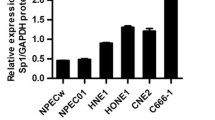
Nasopharyngeal carcinoma (NPC) is one of the aggressive malignant tumors with high mortality, and the proliferation of myeloid-derived suppressor cells (MDSCs) could promote the metastasis of NPC through inhibiting the function of T cells. Meanwhile, SPLUNC1 was known to inhibit the malignant behavior of NPC cells, while the detailed function of SPLUNC1 in LPS-modified immune microenvironment of NPC remains unclear. To assess the impact of SPLUNC1 in immune microenvironment during the progression of NPC, NPC cells were exposed to LPS and then co-cultured with MDSCs for 48 h. RT-qPCR and western blot were performed to evaluate the mRNA and protein level of SPLUNC1, CXCL-2 and CXCR-2, respectively. The level of IL-1β, IL-6, TNF-α, PD-L1, Arg-1 and iNOS were tested by ELISA. Meanwhile, the expression of CD33+ was tested by flow cytometry. The expression of CXCL-2 and CXCR-2 in NPC cells was higher, compared to that in NP69 cells. In contrast, SPLUNC1 level in NPC cells was much lower than that in NP69 cells. SPLUNC1 level was negatively correlated with CXCL-2 and CXCR-2. Overexpression of SPLUNC1 reversed LPS-induced inflammatory responses and proliferation in NPC cells. In addition, SPLUNC1 upregulation could reverse LPS-induced proliferation of MDSCs in tumor microenvironment. Meanwhile, SPLUNC1 overexpression could regulate CXCL-2/CXCR-2 axis through decreasing CXCL-2 and CXCR-2 protein and mRNA expression. SPLUNC1 regulates LPS-induced progression of nasopharyngeal carcinoma and proliferation of MDSCs. Thus, our study might provide a theoretical basis for discovering new strategies against NPC.






Similar content being viewed by others
References
Miao G, Liu B, Ling K, Peng T, Zhou E, Xie S, et al. Long noncoding RNA HCP5 contributes to nasopharyngeal carcinoma progression by targeting microRNA-128-3p. J Oncol. 2022;2022:5740857. https://doi.org/10.1155/2022/5740857.
Zhang X, Liu J, Ji M, Qi G, Qiao R. Long noncoding RNA GUSBP11 knockdown alleviates nasopharyngeal carcinoma via regulating miR-1226-3p/TM9SF4 Axis. Cancer Biother Radiopharm. 2022. https://doi.org/10.1089/cbr.2021.0391.
Liang YL, Zhang Y, Tan XR, Qiao H, Liu SR, Tang LL, et al. A lncRNA signature associated with tumor immune heterogeneity predicts distant metastasis in locoregionally advanced nasopharyngeal carcinoma. Nat Commun. 2022;13(1):2996. https://doi.org/10.1038/s41467-022-30709-6.
Lei Y, Luo W, Gong Q, Luo L, Jing W. Long non-coding RNA cancer susceptibility candidate 9 regulates the malignant biological behavior of nasopharyngeal carcinoma cells by targeting miR-497-5p/Wnt3a/beta-catenin signaling pathway. Front Oncol. 2022;12: 807052. https://doi.org/10.3389/fonc.2022.807052.
Han R, Chen S, Wang J, Zhao Y, Li G. Statement of retraction: LncRNA UCA1 affects epithelial-mesenchymal transition, invasion, migration and apoptosis of nasopharyngeal carcinoma cells. Cell Cycle. 2022;21(8):874. https://doi.org/10.1080/15384101.2022.2046808.
Zhong Q, Chen Y, Chen Z. Statement of Retraction: LncRNA MINCR regulates irradiation resistance in nasopharyngeal carcinoma cells via the microRNA-223/ZEB1 axis. Cell Cycle. 2022;21(8):875. https://doi.org/10.1080/15384101.2022.2046812.
Mashbat EB, Hodeib S, Bidmos F, Thwaites RS, Lu Y, Wright V, Herberg J, Klobassa DS, Zenz W, Hansel TT. Correction to: A rare mutation in SPLUNC1 affects bacterial adherence and invasion in meningococcal disease. Clin Infect Dis. 2022. https://doi.org/10.1093/cid/ciac263.
Jaiswal AK, Yadav J, Makhija S, Sandey M, Suryawanshi A, Mitra AK, et al. Short palate, lung, and nasal epithelial clone 1 (SPLUNC1) level determines steroid-resistant airway inflammation in aging. Am J Physiol Lung Cell Mol Physiol. 2022;322(1):L102–15. https://doi.org/10.1152/ajplung.00315.2021.
Di YP. Functional roles of SPLUNC1 in the innate immune response against Gram-negative bacteria. Biochem Soc Trans. 2011;39(4):1051–5. https://doi.org/10.1042/BST0391051.
Zhang W, Zeng Z, Wei F, Chen P, Schmitt DC, Fan S, et al. SPLUNC1 is associated with nasopharyngeal carcinoma prognosis and plays an important role in all-trans-retinoic acid-induced growth inhibition and differentiation in nasopharyngeal cancer cells. FEBS J. 2014;281(21):4815–29. https://doi.org/10.1111/febs.13020.
Zheng W, Zhu Y, Chen X, Zhao J. CD73 expression in myeloid-derived suppressor cells is correlated with clinical stages in head and neck squamous cell carcinomas. Ann Transl Med. 2021;9(14):1148. https://doi.org/10.21037/atm-21-2589.
Zhang H, Li X, Liao S, Wang H, Chen P, Zhu G, et al. SPLUNC1 knockout enhances LPS-induced lung injury by increasing recruitment of CD11b+Gr-1+ cells to the spleen of mice. Oncol Rep. 2018;39(1):358–66. https://doi.org/10.3892/or.2017.6063.
Acker G, Zollfrank J, Jelgersma C, Nieminen-Kelha M, Kremenetskaia I, Mueller S, et al. The CXCR2/CXCL2 signalling pathway - an alternative therapeutic approach in high-grade glioma. Eur J Cancer. 2020;126:106–15. https://doi.org/10.1016/j.ejca.2019.12.005.
Qiu WZ, Zhang HB, Xia WX, Ke LR, Yang J, Yu YH, et al. The CXCL5/CXCR2 axis contributes to the epithelial-mesenchymal transition of nasopharyngeal carcinoma cells by activating ERK/GSK-3beta/snail signalling. J Exp Clin Cancer Res. 2018;37(1):85. https://doi.org/10.1186/s13046-018-0722-6.
Zhang H, Ye YL, Li MX, Ye SB, Huang WR, Cai TT, et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene. 2017;36(15):2095–104. https://doi.org/10.1038/onc.2016.367.
Liu Y, Di ME, Chu HW, Liu X, Wang L, Wenzel S, et al. Increased susceptibility to pulmonary Pseudomonas infection in Splunc1 knockout mice. J Immunol. 2013;191(8):4259–68. https://doi.org/10.4049/jimmunol.1202340.
Zhang CX, Ye SB, Ni JJ, Cai TT, Liu YN, Huang DJ, et al. STING signaling remodels the tumor microenvironment by antagonizing myeloid-derived suppressor cell expansion. Cell Death Differ. 2019;26(11):2314–28. https://doi.org/10.1038/s41418-019-0302-0.
Schildberger A, Rossmanith E, Eichhorn T, Strassl K, Weber V. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediators Inflamm. 2013;2013: 697972. https://doi.org/10.1155/2013/697972.
Wang F, Zhang H, Qiu G, Li Z, Wang Y. The LINC00452/miR-204/CHST4 axis regulating thymic tregs might be involved in the progression of thymoma-associated myasthenia gravis. Front Neurol. 2022;13: 828970. https://doi.org/10.3389/fneur.2022.828970.
Wrennall JA, Ahmad S, Worthington EN, Wu T, Goriounova AS, Voeller AS, et al. A SPLUNC1 peptidomimetic inhibits Orai1 and reduces inflammation in a murine allergic asthma model. Am J Respir Cell Mol Biol. 2022;66(3):271–82. https://doi.org/10.1165/rcmb.2020-0452OC.
Bian S, Wang Z, Chen Y, Li R. SPLUNC1 and MLL3 regulate cancer stem cells in nasopharyngeal carcinoma. J BUON. 2019;24(4):1700–5.
Tsou YA, Tung YT, Wu TF, Chang GR, Chen HC, Lin CD, et al. Lactoferrin interacts with SPLUNC1 to attenuate lipopolysaccharide-induced inflammation of human nasal epithelial cells via down-regulated MEK1/2-MAPK signaling. Biochem Cell Biol. 2017;95(3):394–9. https://doi.org/10.1139/bcb-2016-0047.
Thaikoottathil JV, Martin RJ, Di PY, Minor M, Case S, Zhang B, et al. SPLUNC1 deficiency enhances airway eosinophilic inflammation in mice. Am J Respir Cell Mol Biol. 2012;47(2):253–60. https://doi.org/10.1165/rcmb.2012-0064OC.
Yu PF, Huang Y, Han YY, Lin LY, Sun WH, Rabson AB, et al. TNFalpha-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2(+) neutrophils. Oncogene. 2017;36(4):482–90. https://doi.org/10.1038/onc.2016.217.
Zhang W, Wang H, Sun M, Deng X, Wu X, Ma Y, et al. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Commun (Lond). 2020;40(2–3):69–80. https://doi.org/10.1002/cac2.12010.
Cheng Y, Mo F, Li Q, Han X, Shi H, Chen S, et al. Targeting CXCR2 inhibits the progression of lung cancer and promotes therapeutic effect of cisplatin. Mol Cancer. 2021;20(1):62. https://doi.org/10.1186/s12943-021-01355-1.
Ou C, Sun Z, Zhang H, Xiong W, Ma J, Zhou M, et al. SPLUNC1 reduces the inflammatory response of nasopharyngeal carcinoma cells infected with the EB virus by inhibiting the TLR9/NF-kappaB pathway. Oncol Rep. 2015;33(6):2779–88. https://doi.org/10.3892/or.2015.3913.
Zeng Y, Cao J, Li CX, Wang CY, Wu RM, Xu XL. MDM2-mediated ubiquitination of RXRbeta contributes to mitochondrial damage and related inflammation in atherosclerosis. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23105766.
Zeng Y, Yan Wang C, Xu J, Le Xu X. Overexpression of retinoid X receptor beta provides protection against oxidized low-density lipoprotein-induced inflammation via regulating PGC1alpha-dependent mitochondrial homeostasis in endothelial cells. Biochem Pharmacol. 2021;188: 114559. https://doi.org/10.1016/j.bcp.2021.114559.
Magierowska K, Korbut E, Wojcik-Grzybek D, Bakalarz D, Sliwowski Z, Cieszkowski J, et al. Mitochondria-targeted hydrogen sulfide donors versus acute oxidative gastric mucosal injury. J Control Release. 2022. https://doi.org/10.1016/j.jconrel.2022.05.051.
Wang D, Cao Q. Shp2 in alveolar macrophages regulates macrophage i phenotype in acute lung injury. Int J Toxicol. 2022. https://doi.org/10.1177/10915818221105227.
Abdel-Hakeem EA, Hafez S, Kamel BA, Abdel-Hamid HA. Angiotensin 1–7 mitigates rhabdomyolysis induced renal injury in rats via modulation of TLR-4/NF-kB/iNOS and Nrf-2/hemeoxygenase-1 signaling pathways. Life Sci. 2022. https://doi.org/10.1016/j.lfs.2022.120678.
Ji J, Zhuang Y, Wang H, Feng C, Zhao Y, Zhang X. M-CSF and prostratin induced Mregs promote immune tolerance in transplanted mice through Arg-1 pathway. Int Immunopharmacol. 2021;99: 108014. https://doi.org/10.1016/j.intimp.2021.108014.
Zhu X, Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget. 2017;8(57):97671–82. https://doi.org/10.18632/oncotarget.18311.
Yuan H, Xue Y, Guo C, Song J, Zhang Y, Yin T, et al. Thermodynamic stability of cisplatin-loaded polymeric micelles and the phenotypic switching of the tumor-associated macrophages induced by combination of cisplatin-loaded micelles and anti-PD-L1 antibody. Int J Pharm. 2022. https://doi.org/10.1016/j.ijpharm.2022.121860.
Recondo G, Mezquita L. Tiragolumab and atezolizumab in patients with PD-L1 positive non-small-cell lung cancer. Lancet Oncol. 2022;23(6):695–7. https://doi.org/10.1016/S1470-2045(22)00261-3.
Wen X, Zeng X, Cheng X, Zeng X, Liu J, Zhang Y, et al. PD-L1-targeted radionuclide therapy combined with alphaPD-L1 antibody immunotherapy synergistically improves the antitumor effect. Mol Pharm. 2022. https://doi.org/10.1021/acs.molpharmaceut.2c00281.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82173201), the Natural Science Foundation of Hunan Province (Grant No. 2021JJ30422), and Hunan Cancer Hospital Climb Plan (Grant No. 2020NSFC-A001). The authors received no financial support for the research, authorship, or publication of this article.
Author information
Authors and Affiliations
Contributions
HW and PC put forward to the hypothesis and revised the manuscript. PC wrote this manuscript. LT, LP and HL performed the part of experiment. Data analysis in the study was conducted by TX, WG and HY. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
These authors declared no competing interests in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, L., Peng, L., Liu, H. et al. SPLUNC1 regulates LPS-induced progression of nasopharyngeal carcinoma and proliferation of myeloid-derived suppressor cells. Med Oncol 39, 214 (2022). https://doi.org/10.1007/s12032-022-01816-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01816-7