Abstract
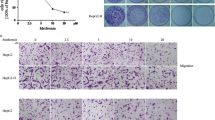
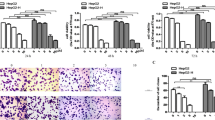
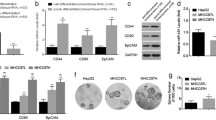
Radiofrequency ablation (RFA) is popularly used in the treatment of hepatocellular carcinoma (HCC). However, the accelerated malignant progression of residual HCC cells after RFA is the main obstacle for the application of this technology in HCC treatment. In the present study, HepG2 cells, an established human HCC cell line, experienced repeatedly with heat treatment, survived cells, HepG2-H cells, were used to simulate residual HCC cells after RFA. The abilities of proliferation, colony formation, and migration were compared between HepG2 and HepG2-H cells. Then, RNA sequencing was used to explore the difference in genes expression between two groups of cells. Subsequently, the level of c-Met, one of membranous receptors of MAPK signal pathway, was measured by RT-qPCR and western blot; the effect of c-Met inhibition on the malignant progression of HepG2-H cells was evaluated. The results showed that HepG2-H cells exhibited higher abilities in the proliferation, colony formation, and migration than that of HepG2 cells. Moreover, differentially expressed genes between two groups of cells were prominently enriched in MAPK signal pathway. The level of c-Met in HepG2-H cells was significantly higher than that in HepG2 cells, and the inhibition in the activity of c-Met could repress the malignant behaviors of HepG2-H cells. These results indicated that the accelerated malignant progression of residual HCC cells after RFA can be partly attributed to the overexpression of c-Met and the activation of MAPK signal pathway. Therefore, we proposed that RFA followed by c-Met inhibitor intake maybe is a better treatment protocol for HCC.






Similar content being viewed by others
Data availability
The raw data used to support the findings of this study are available from the corresponding authors upon request.
References
Che YH, Chongsuvivatwong V, Li L, et al. Financial burden on the families of patients with hepatitis B virus-related liver diseases and the role of public health insurance in Yunnan province of China. Public Health. 2016;130:3–20.
Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249(1):20–5.
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–55.
Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: an updated review. World J Gastrointest Pharmacol Ther. 2016;7(4):477–89.
Obara K, Matsumoto N, Okamoto M, et al. Insufficient radiofrequency ablation therapy may induce further malignant transformation of hepatocellular carcinoma. Hepatol Int. 2008;2(1):116–23.
Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230(1):1–8.
Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–12.
Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802.
Facciorusso A, Del Prete V, Antonino M, et al. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig Liver Dis. 2014;46(11):1014–9.
Yoshida S, Kornek M, Ikenaga N, et al. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2013;58(5):1667–80.
Thompson SM, Callstrom MR, Butters KA, et al. Role for putative hepatocellular carcinoma stem cell subpopulations in biological response to incomplete thermal ablation: in vitro and in vivo pilot study. Cardiovasc Intervent Radiol. 2014;37(5):1343–51.
Wu L, Fu Z, Zhou S, et al. HIF-1α and HIF-2α: siblings in promoting angiogenesis of residual hepatocellular carcinoma after high-intensity focused ultrasound ablation. PLoS ONE. 2014;9(2):e88913.
Kong J, Kong J, Pan B, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PLoS ONE. 2012;7(5):e37266.
Tong R, Wu X, Liu Y, et al. Curcumin-induced DNA demethylation in human gastric cancer cells is mediated by the DNA-damage response pathway. Oxid Med Cell Longev. 2020;2020:2543504.
Zhang Y, Xia M, Jin K, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17(1):45.
Liu Z, Dai H, Jia G, Li Y, Liu X, Ren W. Insufficient radiofrequency ablation promotes human hepatoma SMMC7721 cell proliferation by stimulating vascular endothelial growth factor overexpression. Oncol Lett. 2015;9(4):1893–6.
Ouyang Y, Liu K, Hao M, et al. Radiofrequency ablation-increased CXCL10 is associated with earlier recurrence of hepatocellular carcinoma by promoting stemness. Tumour Biol. 2016;37(3):3697–704.
Zeng J, Cai X, Hao X, et al. LncRNA FUNDC2P4 down-regulation promotes epithelial-mesenchymal transition by reducing E-cadherin expression in residual hepatocellular carcinoma after insufficient radiofrequency ablation. Int J Hyperthermia. 2018;34(6):802–11.
Zhang N, Li H, Qin C, et al. Insufficient radiofrequency ablation promotes the metastasis of residual hepatocellular carcinoma cells via upregulating flotillin proteins. J Cancer Res Clin Oncol. 2019;145(4):895–907.
Li Z, Jiang M, Zhang T, Liu S. GAS6-AS2 promotes hepatocellular carcinoma via miR-3619-5p/ARL2 axis under insufficient radiofrequency ablation condition. Cancer Biother Radiopharm. 2020. https://doi.org/10.1089/cbr.2019.3541.
Kong J, Yao C, Ding X, et al. ATPase inhibitory factor 1 promotes hepatocellular carcinoma progression after insufficient radiofrequency ablation, and attenuates cell sensitivity to sorafenib Therapy. Front Oncol. 2020;10:1080.
Dong S, Kong J, Kong F, et al. Sorafenib suppresses the epithelial-mesenchymal transition of hepatocellular carcinoma cells after insufficient radiofrequency ablation. BMC Cancer. 2015;15:939.
Zhao W, Li X, Li Z. Combination therapy with local radiofrequency ablation and YC-1 inhibits the proliferation and metastasis of hepatocellular carcinoma through activating β-catenin signaling. Pharmazie. 2016;71(9):524–9.
Su T, Liao J, Dai Z, et al. Stress-induced phosphoprotein 1 mediates hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Oncogene. 2018;37(26):3514–27.
Yuan CW, Wang ZC, Liu K, Liu DJ. Incomplete radiofrequency ablation promotes the development of CD133+ cancer stem cells in hepatocellular carcinoma cell line HepG2 via inducing SOX9 expression. Hepatobiliary Pancreat Dis Int. 2018;17(5):416–22.
Tan L, Chen S, Wei G, et al. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019;460:29–40.
Jiang J, Chen S, Li K, et al. Targeting autophagy enhances heat stress-induced apoptosis via the ATP-AMPK-mTOR axis for hepatocellular carcinoma. Int J Hyperthermia. 2019;36(1):499–510.
Xu WL, Wang SH, Sun WB, et al. Insufficient radiofrequency ablation-induced autophagy contributes to the rapid progression of residual hepatocellular carcinoma through the HIF-1α/BNIP3 signaling pathway. BMB Rep. 2019;52(4):277–82.
Chen T, Huang H, Zhou Y, et al. HJURP promotes hepatocellular carcinoma proliferation by destabilizing p21 via the MAPK/ERK1/2 and AKT/GSK3β signaling pathways. J Exp Clin Cancer Res. 2018;37(1):193.
Wang L, Sun L, Wang Y, et al. miR-1204 promotes hepatocellular carcinoma progression through activating MAPK and c-Jun/AP1 signaling by targeting ZNF418. Int J Biol Sci. 2019;15(7):1514–22.
Chen J, Ji T, Wu D, et al. Human mesenchymal stem cells promote tumor growth via MAPK pathway and metastasis by epithelial mesenchymal transition and integrin α5 in hepatocellular carcinoma. Cell Death Dis. 2019;10(6):425.
Imura Y, Nakai T, Yamada S, et al. Functional and therapeutic relevance of hepatocyte growth factor/c-MET signaling in synovial sarcoma. Cancer Sci. 2016;107(12):1867–76.
Wu JC, Wang CT, Hung HC, et al. Heteronemin is a novel c-Met/STAT3 inhibitor against advanced prostate cancer cells. Prostate. 2016;76(16):1469–83.
Pilotto S, Carbognin L, Karachaliou N, et al. Tracking MET de-addiction in lung cancer: a road towards the oncogenic target. Cancer Treat Rev. 2017;60:1–11.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant Nos. 41666007 and 31660340]; Natural Science Foundation of Inner Mongolia Autonomous Region of China [Grant Nos. 2018MS03062 and 2019MS02024].
Author information
Authors and Affiliations
Contributions
LM and XJ (co-corresponding authors) designed the research and wrote the paper; GJ and FL (co-first authors) performed the most of experiments, RT, MZ and YL participated in some of experiments. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Research involving human and animal rights
This article does not contain any research on humans or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jia, G., Li, F., Tong, R. et al. c-Met/MAPK pathway promotes the malignant progression of residual hepatocellular carcinoma cells after insufficient radiofrequency ablation. Med Oncol 37, 117 (2020). https://doi.org/10.1007/s12032-020-01444-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01444-z