Abstract
Genetic heterogeneity is a well-recognized feature of hepatocellular carcinoma (HCC). The coexistence of multiple genetic alterations in the same HCC nodule contributes to explain why gene-targeted therapy has largely failed. Targeting of early genetic alterations could theoretically be a more effective therapeutic strategy preventing HCC. However, the failure of most targeted therapies has raised much perplexity regarding the role of genetic alterations in driving cancer as the main paradigm. Here, we discuss the methodological and conceptual limitations of targeting genetic alterations and their products that may explain the limited success of the novel mechanism-based drugs in the treatment of HCC. In light of these limitations and despite the era of the so-called “precision medicine,” prevention and early diagnosis of conditions predisposing to HCC remain the gold standard approach to prevent the development of this type of cancer. Finally, a paradigm shift to a more systemic approach to cancer is required to find optimal therapeutic solutions to treat this disease.
Similar content being viewed by others
Introduction
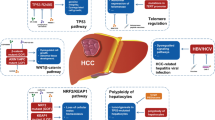
In the last decade, numerous studies have provided several and accurate details on the genetic alterations associated with hepatocellular carcinoma (HCC). In particular, the familial genetic alterations responsible for the development of cirrhosis and associated with HCC formation have been entirely unraveled [1]. Moreover, many efforts have been made to recognize the genetic alterations that are observed in the tumor tissue, since they could be used as potential theranostic targets or could improve decisions/therapies in HCC [1, 2]. However, despite the considerable efforts made in this field and a large number of identified genes (Table 1), the presence of tumor genetic heterogeneity among patients as well as the coexistence of different types of genetic alterations in the same nodule still represent two unsolved problems that explain the limited success of the novel mechanism-based drugs in the treatment of HCC.
As shown in Table 1, a variety of genetic alterations have been reported in HCC. Mutations due to nucleotide substitutions or HBV-DNA insertions leading to an increased telomerase reverse-transcriptase (TERT) gene expression in HCC, with a prevalence of 60–90%, have been reported [3,4,5]. Deletion of exon three, missense mutations, and HBV-DNA insertions in the Cadherin-associated protein β1 (CTNNB1) gene, causing β-catenin and IL6/JAK/STAT activation and the inflammatory response sustenance have been also reported [6,7,8,9,10]. Mutation at codon 249 of the Tumor Protein 53 (TP53) gene has been described in 13–48% of HCC [7,8,9,10,11,12,13,14]. Furthermore, homozygous deletions/mutations or epigenetic silencing of cyclin-dependent kinase inhibitor 2A (CDKN2A) have been shown to contribute to the loss of function of this tumor suppressor gene in HCC [7, 9, 15, 16]. Finally, amplification of vascular endothelial growth factor A (VEGFA) [1, 17,18,19,20] and Fibroblast growth factor 19 (FGF19) gene [21, 22] predisposing to angiogenic and pro-proliferative signaling were found in 7–11% and 6.5% of HCC, respectively.
Based on these considerations, we can assert that the pharmacological principles of potential therapeutic agents targeting genetic alterations have been probably based on the wrong paradigm [23]. First, the presence of multiple genetic alterations itself represents an a priori mechanism of resistance and, theoretically, a barrier hard to overpass in terms of drug design and therapeutic strategy. Second, targeting a single gene or mutation often results in relapse since tumor cells easily escape or compensate for this type of block. Third, it is hard to establish which mutation or alteration is the most relevant to cancer formation or progression in a determined type or subset of HCC. Fourth, the presence of a genetic alteration could not necessarily be considered the driver of cancer promotion but could, instead, represent the consequence of uncontrolled proliferation. Fifth, numerous oncogenic alterations are currently undruggable. Several drugs against genetic alterations or their products have proven ineffective in terms of benefits on overall survival and quality of life in different forms of cancer [24].
The failure of these therapeutic strategies reinforces the importance of conventional therapies (liver transplantation, surgical resection, locoregional therapies), which used alone or in combination, remain the only widely accepted clinical treatments for HCC [25,26,27]. Therefore, the recognition of the subjects at risk, essentially cirrhotics, strictly monitored mainly by echotomography and suitable serum markers (α-fetoprotein), and the initiation of early treatment remain the commonly used strategy to cure, even if it may be considered suboptimal in the era of “precision medicine” [28]. However, in the last years, the publication of a large number of experimental and clinical studies on the possible role of immunotherapy to cure HCC could represent a new promising perspective [29].
An entirely different approach to the cure of HCC could consist of the prevention of the disease through the identification and targeting of the early genetic alterations that anticipate the neoplastic transformation. Unfortunately, this strategy seems also not to succeed in the difficulty to target/correct familial genetic alteration and oncogenic mutations. In this contest, the HBV transgenic mouse model offers the opportunity to study the process of tumor initiation and progression related to the HBV infection [30, 31], and to develop the targeting of the early genetic alterations. Using this model, an early, pre-neoplastic up-regulation of two genes namely cyclin-dependent kinase inhibitor 2D (Cdkn2d) and stromal cell-derived one alpha receptor (CXCR4) was detected [30]. Cdkn2d is expressed in HCC nodules and stimulates cell proliferation, whereas CXCR4 is associated with an immunosuppressive tumor microenvironment [9, 30, 32]. Moreover, using a similar HBV transgenic mouse model, Sun et al. [31] uncovered a set of genes up-regulated in different phases of hepatocarcinogenesis including Trefoil Factor 3 (TFF3), Insulin-like growth factor 2 (IGF2), Lipoprotein Lipase (LPL), and Secreted Phosphoprotein 1 (SPP1, the homolog of human Osteopontin). Finally, Nault et al. found a bi-allelic inactivation of TCF1 in hepatic adenomas, which is related to inactivating mutations of HNF1A [10]. However, further studies are needed to confirm if what found in animal models is translatable to humans.
Another important aspect regarding the development of new potential therapeutic agents against HCC that should be taken into account is the role of metabolic-induced modifications acting as environmental modifiers with the ability to influence a susceptible genetic or epigenetic background [33]. In this context, particular importance is assuming the emerging non-alcoholic fatty liver disease (NAFLD), an association of microenvironmental inflammation, aberrant metabolism, and liver regeneration [33] that could confer an increased risk of HCC, regardless of cirrhosis [34]. Current anti-HCC pharmacological approaches are still unsatisfactory and mainly rely on tyrosine-kinase inhibitors, such as sorafenib [35, 36]. Alpha-fetoprotein (AFP) remains the commonly used diagnostic biomarker since promising markers quite often not directly correlate with the tumor burden [37]. Also, the advanced phase of the neoplastic disease still faces pharmacological challenges [38]. We have been working on identifying novel pharmacological targets and therapeutic strategies in HCC [39,40,41,42]. However, there is still a long way to go in the field of anti-HCC drug strategies. Also, the prevalent paradigm mainly based on the gene-centered theories could not help design a more integrative pharmacology. A systemic approach to cancer may instead be significant to a broad therapeutic approach [23, 43,44,45]. Therefore, HCC prevention or treatment of metabolic conditions including NAFLD still represents an important integrative approach that may probably be more achievable and effective to prevent HCC development and progression, according to the principle of the complexity of systems biology including cancer biology.
References
Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149:1226–9.
Nakatsuka T, Tateishi K, Kudo Y, Yamamoto K, Nakagawa H, Fujiwara H, et al. Impact of histone demethylase KDM3A-dependent AP-1 transactivity on hepatotumorigenesis induced by PI3K activation. Oncogene. 2017;36:6262–71.
Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, Laurent A, Cherqui D, Balabaud C, Zucman-Rossi J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun. 2013;4:2218.
Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V. Liver cancer: approaching a personalized care. J Hepatol. 2015;62:S144–S156.
Nault JC, Villanueva A. Intratumor molecular and phenotypic diversity in hepatocellular carcinoma. Clin Cancer Res. 2015;21:1786–8.
Huang J, Deng Q, Wang Q, et al. Exome sequencing of hepatitis B virus-associated hepatocellular carcinoma. Nat Genet. 2012;44:1117–21.
Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–8.
Kan Z, Zheng H, Liu X, Li S, Barber TD, Gong Z, Gao H, Hao K, Willard MD, Xu J, Hauptschein R, Rejto PA, Fernandez J, Wang G, Zhang Q, Wang B, Chen R, Wang J, Lee NP, Zhou W, Lin Z, Peng Z, Yi K, Chen S, Li L, Fan X, Yang J, Ye R, Ju J, Wang K, Estrella H, Deng S, Wei P, Qiu M, Wulur IH, Liu J, Ehsani ME, Zhang C, Loboda A, Sung WK, Aggarwal A, Poon RT, Fan ST, Wang J, Hardwick J, Reinhard C, Dai H, Li Y, Luk JM, Mao M. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 2013;23:1422–33.
Tian Y, Ou JH. Genetic and epigenetic alterations in hepatitis B virus-associated hepatocellular carcinoma. Virol Sin. 2015;30:85–91.
Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, Bacq Y, Calderaro J, Paradis V, Ramos J, Scoazec JY, et al. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology. 2017;152:880–94.
Takai A, Dang HT, Wang XW. Identification of drivers from cancer genome diversity in hepatocellular carcinoma. Int J Mol Sci. 2014;15:11142–60.
Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinoma identifies new mutations signatures and potential therapeutic targets. Nat Genet. 2015;47:505–11.
Yamamoto S, Iwakuma T. Regulators of oncogenic mutant TP53 gain of function. Cancers (Basel). 2018;11:E4. https://doi.org/10.3390/cancers11010004.
Long J, Wang A, Bai Y, Lin J, Yang X, Wang D, Yang X, Jiang Y, Zhao H. Development and validation of a TP53-associated immune prognostic model for hepatocellular carcinoma. EBioMedicine. 2019;42:363–74. https://doi.org/10.1016/j.ebiom.2019.03.022.
Totoki Y, Tatsuno K, Covington KR, et al. Transancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46:1267–73.
Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–11.
Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Solé M, Tovar V, Alsinet C, Ramos AH, Barretina J, Roayaie S, Schwartz M, Waxman S, Bruix J, Mazzaferro V, Ligon AH, Najfeld V, Friedman SL, Sellers WR, Meyerson M, Llovet JM. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–888.
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018.
Oh CR, Kong SY, Im HS, Kim HJ, Kim MK, Yoon KA, Cho EH, Jang JH, Lee J, Kang J, Park SR, Ryoo BY. Genome-wide copy number alteration and VEGFA amplification of circulating cell-free DNA as a biomarker in advanced hepatocellular carcinoma patients treated with sorafenib. BMC Cancer. 2019;19:292.
Horwitz E, Stein I, Andreozzi M, Nemeth J, Shoham A, Pappo O, Schweitzer N, Tornillo L, Kanarek N, Quagliata L, Zreik F, Porat RM, Finkelstein R, Reuter H, Koschny R, Ganten T, Mogler C, Shibolet O, Hess J, Breuhahn K, Grunewald M, Schirmacher P, Vogel A, Terracciano L, Angel P, Ben-Neriah Y, Pikarsky E. Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov. 2014;4:730–43.
Raja A, Park I, Haq F, Ahn SM. FGF19–FGFR4 Signaling in hepatocellular carcinoma. Cells. 2019;8:E536. https://doi.org/10.3390/cells8060536.
https://www.cbioportal.org/study/summary?id=lihc_tcga (download February 2020)
Mazzocca A, Ferraro G, Misciagna G, Fais S. Moving the systemic evolutionary approach to cancer forward: therapeutic implications. Med Hypotheses. 2018;9:121. https://doi.org/10.1016/j.mehy.2018.09.033.
Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009–2013. BMJ. 2017;4:359. https://doi.org/10.1136/bmj.j4530.
Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: our experience and published work review. Hepatol Res. 2015;45:59–74.
Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M. Transarterial chemoembolization: evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol. 2015;7:2009–19. https://doi.org/10.4254/wjh.v7.i16.2009.
Facciorusso A, Mariani L, Sposito C, Spreafico C, Bongini M, Morosi C, et al. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:645–53. https://doi.org/10.1016/j.dld.2016.02.005.
Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526–49. https://doi.org/10.1016/j.jhep.2017.09.016.
Marquardt JU, Saborowski A, Czauderna C, Vogel A. The changing landscape of systemic treatment of advanced hepatocellular carcinoma: new targeted agents and immunotherapies. Target Oncol. 2019;14:115–23.
Barone M, Spano D, D'Apolito M, Centra M, Lasalandra M, Capasso M, et al. Gene expression analysis in HBV transgenic mouse liver: a model to study early events related to hepatocarcinogenesis. Mol Med. 2006;12:115–23. https://doi.org/10.2119/2006-00015.
Sun Q, Zhang Y, Liu F, Zhao X, Yang X. Identification of candidate biomarkers for hepatocellular carcinoma through pre-cancerous expression analysis in an HBx transgenic mouse. Cancer Biol Ther. 2007;6:1532–8. https://doi.org/10.4161/cbt.6.10.4683.
Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61:1591–602. https://doi.org/10.1002/hep.27665.
Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–28. https://doi.org/10.1038/s41575-019-0145-7.
Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016;63:827–38. https://doi.org/10.1002/hep.28368.
Gadaleta-Caldarola G, Infusino S, Divella R, Ferraro E, Mazzocca A, De Rose F, Filippelli G, Abbate I, Brandi M. Sorafenib: 10 years after the first pivotal trial. Future Oncol. 2015;11:1863–80. https://doi.org/10.2217/fon.15.85.
Gadaleta-Caldarola G, Divella R, Mazzocca A, Infusino S, Ferraro E, Filippelli G, Daniele A, Sabbà C, Abbate I, Brandi M. Sorafenib: the gold standard therapy in advanced hepatocellular carcinoma and beyond. Future Oncol. 2015;11:2263–6. https://doi.org/10.2217/fon.15.161.
Trerotoli P, Fransvea E, Angelotti U, Antonaci G, Lupo L, Mazzocca A, Mangia A, Antonaci S, Giannelli G. Tissue expression of squamous cellular carcinoma antigen (SCCA) is inversely correlated to tumor size in hepatocellular carcinoma. Mol Cancer. 2009;8:29.
Mazzocca A, Carloni V. The metastatic process: methodological advances and pharmacological challenges. Curr Med Chem. 2009;16:1704–17.
Mazzocca A, Giusti S, Hamilton AD, Sebti SM, Pantaleo P, Carloni V. Growth inhibition by the farnesyltransferase inhibitor FTI-277 involves Bcl-2 expression and defective association with Raf-1 in liver cancer cell lines. Mol Pharmacol. 2003;63:159–66.
Mazzocca A, Dituri F, De Santis F, Filannino A, Lopane C, Betz RC, Li YY, Mukaida N, Winter P, Tortorella C, Giannelli G, Sabbà C. Lysophosphatidic acid receptor LPAR6 supports the tumorigenicity of hepatocellular carcinoma. Cancer Res. 2015;1(75):532–43.
Gnocchi D, Kapoor S, Nitti P, Cavalluzzi MM, Lentini G, Denora N, Sabbà C, Mazzocca A. Novel lysophosphatidic acid receptor 6 antagonists inhibit hepatocellular carcinoma growth through affecting mitochondrial function. J Mol Med (Berl). 2020;98:179–91.
Turato C, Balasso A, Carloni V, Tiribelli C, Mastrotto F, Mazzocca* A, Pontisso P. New molecular targets for functionalized nanosized drug delivery systems in personalized therapy for hepatocellular carcinoma. J Control Release. 2017;268:184–97. https://doi.org/10.1016/j.jconrel.2017.10.027.
Sonnenschein C, Soto AM, Rangarajan A, Kulkarni P. Competing views on cancer. J Biosci. 2014;39:281–302.
Mazzocca A, Ferraro G, Misciagna G, Carr BI. A systemic evolutionary approach to cancer: hepatocarcinogenesis as a paradigm. Med Hypotheses. 2016;93:132–7.
Mazzocca A. The systemic-evolutionary theory of the origin of cancer: a new interpretative model of cancer as a complex biological system. Int J Mol Sci. 2019;20(19):4885.
Acknowledgements
Open access funding provided by Università degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement.
Funding
This paper was partly funded by the Italian Ministry of University and Research—PON-BIOMIS 2018 (Project: Costituzione della Biobanca del Microbiota intestinale e salivare umano: dalla disbiosi alla simbiosi). This work was also supported by a research grant from AIRC (Italian Association for Cancer Research) to A. Mazzocca [Investigator Grant (IG) 2015 Id.17758].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by MB and AM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript and gave explicit consent to the submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barone, M., Di Leo, A., Sabbà, C. et al. The perplexity of targeting genetic alterations in hepatocellular carcinoma. Med Oncol 37, 67 (2020). https://doi.org/10.1007/s12032-020-01392-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01392-8