Abstract
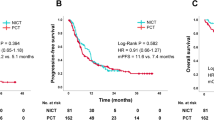
A phase I study recommended carboplatin (CBDCA, area under the curve = 5) plus pemetrexed (PEM, 500 mg/m2) for elderly patients (≥75-years old) with non-squamous non-small cell lung cancer (NSCLC). PEM maintenance therapy was well tolerated. We conducted a multicenter phase II trial to evaluate the efficacy and safety of this regimen in elderly patients with NSCLC. Four courses of CBDCA plus PEM, followed by PEM, were administered. The primary endpoint was the 1-year overall survival (OS) rate, and the secondary endpoints were OS, progression-free survival (PFS), response rate (RR), and safety. Thirty-four patients (median age, 77 years) were enrolled between June 2012 and May 2013. The median observation time was 22.7 months. The primary endpoint of the 1-year OS rate was 58.0 % (95 % confidence interval (CI) 42.9–78.4 %), and RR and disease control rate were 41.2 and 85.3 %, respectively. Fourteen patients had partial responses, 15 had stable disease, 4 had disease progression, and 1 was not evaluated. The maintenance therapy rate was 58.8 %. The median PFS was 5.7 months (95 % CI 3.9–8.9 months), and median OS was 20.5 months (95 % CI 10.0–infinity months). Grade ≥3 hematological adverse events included leucopenia (23.5 % of patients), neutropenia (55.9 %), anemia (35.3 %), and thrombocytopenia (20.6 %). Grade ≥3 non-hematological adverse events included febrile neutropenia (8.8 %), elevated aminotransferases (5.9 %), infection (23.5 %), and anorexia/fatigue (5.9 %). Four patients had interstitial lung diseases (ILD) and one died due to ILD. CBDCA plus PEM, followed by PEM, was effective and reasonably tolerated in chemotherapy-naïve elderly patients with non-squamous NSCLC.
Clinical Trial Registration This trial is registered with the University Hospital Medical Information Network Clinical Trials Registry (Trial Number UMIN 000004810).


Similar content being viewed by others
References
National Cancer Institute: SEER Stat Fact Sheets: http://seer.cancer.gov/statfacts/html/lungb.html.
Gridelli C, Langer C, Maione P, et al. Lung cancer in the elderly. J Clin Oncol. 2007;25:1898–907.
Gridelli C, Rossi A, Maione P. Challenges treating older non-small cell lung cancer patients. Ann Oncol. 2008; 19: vii 109–113.
Hoffe S, Balducci L. Cancer and age: general considerations. Clin Geriatr Med. 2012;28:1–18.
Gridelli C, Balducci L, Ciardiello F, et al. Treatment of elderly patients with non-small cell lung cancer: results of an international expert panel meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer. 2015;16:325–33.
Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909.
Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol. 1997;15:2996–3018.
Socinski MA, Crowell R, Hensing TE, et al. Treatment of non-small cell lung cancer, stage IV: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:277S–89S.
Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small cell lung cancer. J Clin Oncol. 2008;26:3543–51.
Scagliotti GV, Hanna N, Fossella FV, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist. 2009;14:253–63.
Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–55.
Jennens RR, Giles GG, Fox RM. Increasing underrepresentation of elderly patients with advanced colorectal or non-small-cell lung cancer in chemotherapy trials. Intern Med J. 2006;36:216–20.
Yee KW, Pater JL, Pho L, et al. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21:1618–23.
Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7.
Quoix E, Zalcman G, Oster J, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–88.
Abe T, Takeda K, Ohe Y, et al. Randomized phase III trial comparing weekly docetaxel plus cisplatin versus docetaxel monotherapy every 3 weeks in elderly patients with advanced non-small-cell lung cancer: the intergroup trial JCOG0803/WJOG4307L. J Clin Oncol. 2015;33:575–81.
Tamiya A, Tamiya M, Shiroyama T, et al. Dose escalation study of carboplatin-pemetrexed followed by maintenance pemetrexed for elderly patients with advanced nonsquamous nonsmall-cell lung cancer. Ann Oncol. 2013;24:980–5.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Grønberg BH, Bremnes RM, Fløtten O, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:3217–24.
Gridelli C. The ELVIS trial: a phase III study of single-agent vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer. Elderly Lung Cancer Vinorelbine Italian Study. Oncologist. 2001;6:4–7.
Kudoh S, Takeda K, Nakagawa K, et al. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group trial (WJOG 9904). J Clin Oncol. 2006;24:3657–63.
Hainsworth JD, Burris HA, Litchy S, et al. Weekly docetaxel in the treatment of elderly patients with advanced nonsmall cell lung carcinoma. A Minnie Pearl Cancer Research Network Phase II Trial. Cancer. 2000;89:328–33.
Fidias P, Supko JG, Martins R, et al. A phase II study of weekly paclitaxel in elderly patients with advanced non-small cell lung cancer. Clin Cancer Res. 2001;7:3942–9.
Okamoto I, Aoe K, Kato T, et al. Pemetrexed and carboplatin followed by pemetrexed maintenance therapy in chemo-naïve patients with advanced nonsquamous non-small-cell lung cancer. Invest New Drugs. 2013;31:1275–82.
Acknowledgments
The authors wish to thank all the participating patients and all the cooperating colleges.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kiyotaka Yoh, Koji Takeda, and Shinji Atagi have received honoraria from Eli Lilly. Hiroaki Okamoto is currently receiving grants from Bristol Myers Squibb. Hirsoshi Tanaka, Kiyotaka Yoh, Kazuhiko Nakagawa, and Shinji Atagi are currently receiving grants from Eli Lilly. The remaining authors do not declare any conflicts of interest.
Rights and permissions
About this article
Cite this article
Tamiya, M., Tamiya, A., Kaneda, H. et al. A phase II study of pemetrexed plus carboplatin followed by maintenance pemetrexed as first-line chemotherapy for elderly patients with advanced non-squamous non-small cell lung cancer. Med Oncol 33, 2 (2016). https://doi.org/10.1007/s12032-015-0715-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0715-7