Abstract
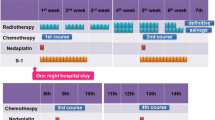
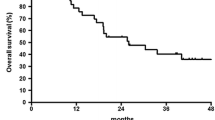
Adding docetaxel to the cisplatin/5-fluorouracil induction regimen for locally advanced esophageal and GEJ cancer may increase the pathologic complete remission (pCR) rate, leading to an improved outcome. Institutional ethics committee approved the protocol of retrospective analysis of patients with locally advanced esophageal and GEJ carcinoma, who received 2–3 cycles of docetaxel, cisplatin and 5-fluorouracil (DCF) induction chemotherapy with primary growth factors and prophylactic antibiotics. Following chemotherapy, a restaging scan was performed. If disease was deemed resectable, surgery was performed. Between February 2010 and October 2013, 31 patients received induction DCF. Ninety-four percent patients had squamous histology. Response rate was 81 %: complete remission (CR)—23 % and partial remission—58 %. Eighty-seven percent patients underwent surgery; R0 resection rate was 67 %. pCR occurred in 26 %. Common grade 3/4 toxicities included anemia—23 %, neutropenia—42 %, febrile neutropenia—39 %, diarrhea—39 %, hyponatremia—55 % and hypokalemia—39 %. There were no toxic deaths. At a median follow-up of 34 months (95 % CI 31.3–36.6), estimated median progression-free survival (PFS) was 27 months (95 % CI 11–39) and the overall survival (OS) at 1 year, 2 years and 3 years was 80, 68 and 55 %, respectively. Patients who attained pCR had a significant longer PFS and OS; median PFS and OS were not reached in patients with pCR and were 15 months (95 %CI 8.4–21.5 months), P = 0.012 and 25 months (95 %CI 10.3–39.7), P = 0.023, respectively, in patients who did not attain a pCR. DCF induction chemotherapy leads to pCR of 26 %, which rivals that obtained from chemoradiotherapy. Toxicity is substantial but manageable with adequate supportive care.




Similar content being viewed by others
References
D’Amico TA. Outcomes after surgery for esophageal cancer. Gastrointest Cancer Res. 2007;1:188–96.
Malthaner R, Fenlon D. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev 2003;(4):CD001556.
Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–33.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, the MAGIC Trial Participants, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Boonstra JJ, Kok TC, Wijnhoven BP, van Heijl M, van Berge Henegouwen MI, Ten Kate FJ, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer. 2011;11:181.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7.
Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, et al. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol. 2012;23:1512–7.
Bayraktar UD, Bayraktar S, Hosein P, Chen E, Koniaris LG, Rocha-Lima CM, et al. Preoperative docetaxel/cisplatin/5-fluorouracil chemotherapy in patients with locally advanced gastro-esophageal adenocarcinoma. Med Oncol. 2012;29:1707–10.
Ui T, Fujii H, Hosoya Y, Nagase M, Mieno MN, Mori M, et al. Comparison of preoperative chemotherapy using docetaxel, cisplatin and fluorouracil with cisplatin and fluorouracil in patients with advanced carcinoma of the thoracic esophagus. Dis Esophagus 2014 Feb 17 [epub ahead of print] doi:10.1111/dote.12187.
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma Clinicopathologic correlations. Cancer. 1994;73:2680–6.
Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, et al. Japan Esophageal Oncology Group/Japan Clinical Oncology Group. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol. 2013;43:752–5.
Fields RC, Strong VE, Gonen M, Goodman KA, Rizk NP, Kelsen DP, et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastroesophageal adenocarcinoma. Br J Cancer. 2011;104:1840–7.
Donahue JM, Nichols FC, Li Z, Schomas DA, Allen MS, Cassivi SD, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2012;93:381–7.
Ancona E, Ruol A, Santi S, Merigliano S, Sileni VC, Koussis H, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165–74.
Stahl M, Wal MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–6.
Van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, CROSS Group, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg. 2014;101:321–38.
Yoshida N, Watanabe M, Baba Y, Ishimoto T, Iwagami S, Sakamoto Y, et al. Influence of preoperative docetaxel, cisplatin, and 5-fluorouracil on the incidence of complications after esophagectomy for resectable advanced esophageal cancer. Dis Esophagus. 2014;27:374–9.
Available online at: http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Last accessed on 6 May 2014.
Ancona E, Ruol A, Castoro C, Chiarion-Sileni V, Merigliano S, Santi S, et al. First-line chemotherapy improves the resection rate and long-term survival of locally advanced (T4, any N, M0) squamous cell carcinoma of the thoracic esophagus: final report on 163 consecutive patients with 5-year follow-up. Ann Surg. 1997;226:714–23.
Miyata H, Yamasaki M, Kurokawa Y, Takiguchi S, Nakajima K, Fujiwara Y, et al. Clinical relevance of induction triplet chemotherapy for esophageal cancer invading adjacent organs. J Surg Oncol. 2012;106:441–7.
Yokota T, Hatooka S, Ura T, Abe T, Takahari D, Shitara K, et al. Docetaxel plus 5-fluorouracil and cisplatin (DCF) induction chemotherapy for locally advanced borderline-resectable T4 esophageal cancer. Anticancer Res. 2011;31:3535–41.
Ilson DH. Docetaxel, cisplatin, and fluorouracil in gastric cancer: does the punishment fit the crime? J Clin Oncol. 2007;25:188–90.
Toh Y, Oki E, Minami K, Okamura T. Follow-up and recurrence after a curative esophagectomy for patients with esophageal cancer: the first indicators for recurrence and their prognostic values. Esophagus. 2010;7:37–43.
Conflict of interest
None.
Ethical standard
All patients included in this study received standard treatment, for which patients gave a written informed consent. Data were prospectively entered into a database. The institutional ethics committee (IEC) of Tata Memorial Hospital, Mumbai, India, approved the analysis of the data included in this database, and the IEC deemed that informed consent was not necessary for the analysis and hence granted a waiver of consent. The study was performed according to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noronha, V., Joshi, A., Jandyal, S. et al. High pathologic complete remission rate from induction docetaxel, platinum and fluorouracil (DCF) combination chemotherapy for locally advanced esophageal and junctional cancer. Med Oncol 31, 188 (2014). https://doi.org/10.1007/s12032-014-0188-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0188-0