Abstract
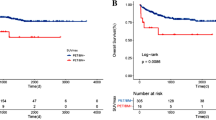
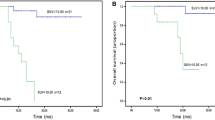
The role of 18Fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) in extranodal natural killer/T-cell lymphoma (ENKL) is not well established. This study aimed to investigate the prognostic role of the pretreatment maximum standardized uptake value (SUVmax) on PET/CT in patients with newly diagnosed ENKL. Among 364 consecutive patients with newly diagnosed ENKL, 81 patients were included and reviewed. The impact of SUVmax on survival and the relationship between SUVmax and other clinicopathological parameters were analyzed. The median SUVmax was 14.6 (range 2.0–45.4). The optimal cutoff value of SUVmax to predict overall survival (OS) was 15. Patients with high SUVmax (SUVmax >15) were associated with bulky disease (P < 0.001), local invasion (P = 0.030), high score of Korean Prognostic Index (KPI, P = 0.046), resistance to primary treatment (P = 0.014), poor OS (P < 0.001), and unfavorable progression-free survival (P < 0.001). With a median follow-up of 25.0 months, the median OS was 63.0 months (range 2.0–99.0 months). Multivariate analyses revealed the following independent prognostic factors for OS: age >60 years (P = 0.001), stage III–IV (P = 0.023), SUVmax >15 (P = 0.020), and bulky disease (>5 cm) (P = 0.002). By using the SUVmax, patients in most subgroups stratified by the KPI or the International Prognostic Index (IPI) were further discriminated in OS with significant statistical difference. Our results suggest the pretreatment SUVmax is predictive of prognosis in patients with newly diagnosed ENKL. The SUVmax may provide additional prognostic information for IPI and KPI.




Similar content being viewed by others
References
Aozasa K, Ohsawa M, Tajima K, et al. Nation-wide study of lethal mid-line granuloma in Japan: frequencies of wegener’s granulomatosis, polymorphic reticulosis, malignant lymphoma and other related conditions. Int J Cancer. 1989;44(1):63–6.
Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–30.
Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835–49.
Jaffe ES, Chan JK, Su IJ, et al. Report of the workshop on nasal and related extranodal angiocentric T/natural killer cell lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol. 1996;20(1):103–11.
Lee J, Kim WS, Park YH, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92(7):1226–30.
Lee J, Park YH, Kim WS, et al. Extranodal nasal type NK/T-cell lymphoma: elucidating clinical prognostic factors for risk-based stratification of therapy. Eur J Cancer. 2005;41(10):1402–8.
Chim CS, Ma SY, Au WY, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood. 2004;103(1):216–21.
Lim ST, Hee SW, Quek R, et al. Comparative analysis of extra-nodal NK/T-cell lymphoma and peripheral T-cell lymphoma: significant differences in clinical characteristics and prognosis. Eur J Haematol. 2008;80(1):55–60.
Oshimi K, Kawa K, Nakamura S, et al. NK-cell neoplasms in Japan. Hematology. 2005;10(3):237–45.
Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24(4):612–8.
Kim TM, Lee SY, Jeon YK, et al. Clinical heterogeneity of extranodal NK/T-cell lymphoma, nasal type: a national survey of the Korean Cancer Study Group. Ann Oncol. 2008;19(8):1477–84.
Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113(17):3931–7.
Au WY, Pang A, Choy C, et al. Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood. 2004;104(1):243–9.
Kim SJ, Kim BS, Choi CW, et al. Ki-67 expression is predictive of prognosis in patients with stage I/II extranodal NK/T-cell lymphoma, nasal type. Ann Oncol. 2007;18(8):1382–7.
Lee J, Suh C, Huh J, et al. Effect of positive bone marrow EBV in situ hybridization in staging and survival of localized extranodal natural killer/T-cell lymphoma, nasal-type. Clin Cancer Res. 2007;13(11):3250–4.
Yasuda H, Sugimoto K, Imai H, et al. Expression levels of apoptosis-related proteins and Ki-67 in nasal NK/T-cell lymphoma. Eur J Haematol. 2009;82(1):39–45.
Juweid ME, Hoekstra OS. Positron emission tomography. New York: Humana Press; 2011.
Cronin CG, Swords R, Truong MT, et al. Clinical utility of PET/CT in lymphoma. AJR Am J Roentgenol. 2010;194(1):W91–103.
Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86.
Juweid ME, Cheson BD. Role of positron emission tomography in lymphoma. J Clin Oncol. 2005;23(21):4577–80.
Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25(5):571–8.
Kelloff GJ, Sullivan DM, Wilson W, et al. FDG-PET lymphoma demonstration project invitational workshop. Acad Radiol. 2007;14(3):330–9.
Seam P, Juweid ME, Cheson BD. The role of FDG-PET scans in patients with lymphoma. Blood. 2007;110(10):3507–16.
Ahmadzadehfar H, Rodrigues M, Zakavi R, et al. Prognostic significance of the standardized uptake value of pre-therapeutic (18)F-FDG PET in patients with malignant lymphoma. Med Oncol. 2011;28(4):1570–6.
Chihara D, Oki Y, Onoda H, et al. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int J Hematol. 2011;93(4):502–8.
Fujiwara H, Maeda Y, Nawa Y, et al. The utility of positron emission tomography/computed tomography in the staging of extranodal natural killer/T-cell lymphoma. Eur J Haematol. 2011;87(2):123–9.
Kako S, Izutsu K, Ota Y, et al. FDG-PET in T-cell and NK-cell neoplasms. Ann Oncol. 2007;18(10):1685–90.
Karantanis D, Subramaniam RM, Peller PJ, et al. The value of [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography in extranodal natural killer/T-cell lymphoma. Clin Lymphoma Myeloma. 2008;8(2):94–9.
Chan WK, Au WY, Wong CY, et al. Metabolic activity measured by F-18 FDG PET in natural killer-cell lymphoma compared to aggressive B- and T-cell lymphomas. Clin Nucl Med. 2010;35(8):571–5.
Suh C, Kang YK, Roh JL, et al. Prognostic value of tumor 18F-FDG uptake in patients with untreated extranodal natural killer/T-cell lymphomas of the head and neck. J Nucl Med. 2008;49(11):1783–9.
Kim TM, Park YH, Lee SY, et al. Local tumor invasiveness is more predictive of survival than International Prognostic Index in stage I(E)/II(E) extranodal NK/T-cell lymphoma, nasal type. Blood. 2005;106(12):3785–90.
Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–77.
Dickinson M, Hoyt R, Roberts AW, et al. Improved survival for relapsed diffuse large B cell lymphoma is predicted by a negative pre-transplant FDG-PET scan following salvage chemotherapy. Br J Haematol. 2010;150(1):39–45.
Horning SJ, Juweid ME, Schoder H et al. Interim positron emission tomography scans in diffuse large B-cell lymphoma: an independent expert nuclear medicine evaluation of the Eastern Cooperative Oncology Group E3404 study. Blood. 2010;115(4):775–777; quiz 918.
Mikhaeel NG, Hutchings M, Fields PA, et al. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16(9):1514–23.
Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25(24):3746–52.
Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107(1):52–9.
Hutchings M, Mikhaeel NG, Fields PA, et al. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol. 2005;16(7):1160–8.
de Wit M, Bohuslavizki KH, Buchert R, et al. 18FDG-PET following treatment as valid predictor for disease-free survival in Hodgkin’s lymphoma. Ann Oncol. 2001;12(1):29–37.
Cheung MM, Chan JK, Lau WH, et al. Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys. 2002;54(1):182–90.
You JY, Chi KH, Yang MH, et al. Radiation therapy versus chemotherapy as initial treatment for localized nasal natural killer (NK)/T-cell lymphoma: a single institute survey in Taiwan. Ann Oncol. 2004;15(4):618–25.
Acknowledgments
We thank Ya-Rui Zhang for her excellent support on data collection.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bing Bai, Hui-Qiang Huang and Qi-Chun Cai contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bai, B., Huang, HQ., Cai, QC. et al. Predictive value of pretreatment positron emission tomography/computed tomography in patients with newly diagnosed extranodal natural killer/T-cell lymphoma. Med Oncol 30, 339 (2013). https://doi.org/10.1007/s12032-012-0339-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-012-0339-0