Abstract
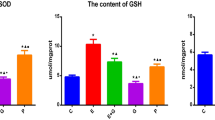
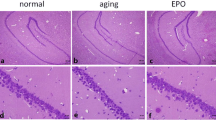
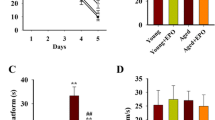
Erythropoietin (EPO) may protect the nervous system of animals against aging damage, making it a potential anti-aging drug for the nervous system. However, experimental evidence from natural aging nerve cell models is lacking, and the efficacy of EPO and underlying mechanism of this effect warrant further study. Thus, the present study used long-term cultured primary nerve cells to successfully mimic the natural aging process of nerve cells. Starting on the 11th day of culture, cells were treated with different concentrations of recombinant human erythropoietin (rhEPO). Using double immunofluorescence labeling, we found that rhEPO significantly improved the morphology of long-term cultured primary nerve cells and increased the total number of long-term cultured primary cells. However, rhEPO did not improve the ratio of nerve cells. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to measure nerve cell activity and showed that rhEPO significantly improved the activity of long-term cultured primary nerve cells. Moreover, Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) double immunofluorescence labeling flow cytometry revealed that rhEPO reduced the apoptotic rate of long-term cultured primary nerve cells. Senescence-associated β-galactosidase (SA-β-gal) immunohistochemistry staining showed that rhEPO significantly reduced the aging rate of long-term cultured primary nerve cells. Immunochemistry revealed that rhEPO enhanced intracellular superoxide dismutase (SOD) activity and glutathione (GSH) abundance and reduced the intracellular malondialdehyde (MDA) level. In addition, this effect depended on the dose, was maximized at a dose of 100 U/ml and was more pronounced than that of vitamin E. In summary, this study finds that rhEPO protects long-term cultured primary nerve cells from aging in a dose-dependent manner. The mechanism of this effect may be associated with the enhancement of the intracellular anti-oxidant capacity. These findings provide a theoretical basis to further the anti-aging mechanism of EPO in the nervous system, and they provide experimental evidence at the cellular level for the clinical application of EPO to protect the nervous system from aging.






Similar content being viewed by others
References
Aksenova MV, Aksenov MY, Markesbery WR, Butterfield DA (1999) Aging in a dish: age-dependent changes of neuronal survival, protein oxidation, and creatine kinase BB expression in long-term hippocampal cell culture. J Neurosci Res 58:308–317
Alcedo J, Flatt T, Pasyukova EG (2013) The role of the nervous system in aging and longevity. Front Genet 4:124
Andrews TJ (1996) Autonomic nervous system as a model of neuronal aging: the role of target tissues and neurotrophic factors. Microsc Res Tech 35:2–19
Belrose JC, Xie YF, Gierszewski LJ, MacDonald JF, Jackson MF (2012) Loss of glutathione homeostasis associated with neuronal senescence facilitates TRPM2 channel activation in cultured hippocampal pyramidal neurons. Mol Brain 5:11
Bertrand SJ, Aksenova MV, Aksenov MY, Charles FM, Rosemarie MB (2011) Endogenous amyloidogenesis in long-term rat hippocampal cell cultures. BMC Neurosci 12:38
Brewer GJ (1995) Serum-free B27/Neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res 42:674–683
Brierley EJ, Johnson MA, Lightowlers RN, James OF, Turnbull DM (1998) Role of mitochondrial DNA mutations in human aging: implications for the central nervous system and muscle. Ann Neurol 43:217–223
Brody H (1955) Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol 102:511–516
Carrasco-Garcia E, Arrizabalaga O, Serrano M, Lovell-Badge R, Matheu A (2015) Increased gene dosage of Ink4/Arf and p53 delays age-associated central nervous system functional decline. Aging Cell 14:710–714
Castañeda-Arellano R, Feria-Velasco AI, Rivera-Cervantes MC (2014) Activity increase in EpoR and Epo expression by intranasal recombinant human erythropoietin (rhEPO) administration in ischemic hippocampi of adult rats. Neurosci Lett 583:16–20
Cervellini I, Ghezzi P, Mengozzi M (2013) Therapeutic efficacy of erythropoietin in experimental autoimmune encephalomyelitis in mice, a model of multiple sclerosis. Methods Mol Biol 982:163–173
Chong ZZ, Shang YC, Mu YL, Cui SX, Yao QQ, Maiese K (2013) Targeting erythropoietin for chronic neurodegenerative diseases. Expert Opin Ther Targets 17:707–720
Chung YH, Kim SI, Joo KM, Kim YS, Lee WB, Yun KW, Cha CI (2004) Age-related changes in erythropoietin immunoreactivity in the cerebral cortex and hippocampus of rats. Brain Res 1018:141–146
Cutler RG (1991) Antioxidant and aging. Am J Clin Nutr 53:373S–379S
Dong W, Cheng S, Huang F, Fan W, Chen Y, Shi H, He H (2011) Mitochondrial dysfunction in long-term neuronal cultures mimics changes with aging. Med Sci Monit 17:BR91–BR96
Dong CM, Wang XL, Wang GM, Zhang WJ, Zhu L, Gao S, Yang DJ, Qin Y, Liang QJ, Chen YL, Deng HT, Ning K, Liang AB, Gao ZL, Xu J (2014) A stress-induced cellular aging model with postnatal neural stem cells. Cell Death Dis 5:e1116
Ermini F, Jucker M (2000) Nerve cell loss and neurogenesis in the aging brain. Praxis (Bern 1994) 89:1605–1608
Hervonen A (1990) The aging nerve cell. Duodecim 106:1497–1500
Ji LL (1993) Antioxidant enzyme response to exercise and aging[J]. Med Sci Sports Exerc 25:225–231
Jia Y, Mo SJ, Feng QQ, Zhan ML, OuYang LS, Chen JC, Ma YX, Wu JJ, Lei WL (2014) EPO-dependent activation of PI3K/Akt/FoxO3a signalling mediates neuroprotection in in vitro and in vivo models of Parkinson’s disease. J Mol Neurosci 53:117–124
Juul S (2002) Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr Suppl 91:36–42
Klichko VI, Radyuk SN, Orr WC (1999) CuZn-SOD promoter-driven expression in the drosophila central nervous system. Neurobiol Aging 20:537–543
Lesuisse C, Martin LJ (2002) Long-term culture of mouse cortical neurons as a model for neuronal development, aging, and death. J Neurobiol 51:9–23
Limke TL, Rao MS (2002) Neural stem cells in aging and disease. J Cell Mol Med 6:475–496
Omelyanchuk LV, Shaposhnikov MV, Moskalev AA (2015) Drosophila nervous system as a target of aging and anti-aging interventions. Front Genet 6:89
Pannese E (2011) Morphological changes in nerve cells during normal aging. Brain Struct Funct 216:85–89
Patel R, Sesti F (2016) Oxidation of ion channels in the aging nervous system. Brain Res 1639:174
Rikans LE, Hornbrook KR (1997) Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta 1362:116–127
Roberts DE, Killiany RJ, Rosene DL (2012) Neuron numbers in the hypothalamus of the normal aging rhesus monkey: stability across the adult lifespan and between the sexes. J Comp Neurol 520:1181–1197
Shoemaker CB, Mitsock LD (1986) Murine erythropoietin gene: cloning, expression, and human gene homology. Mol Cell Biol 6:849–858
Sims-Robinson C, Hur J, Hayes JM, Dauch JR, Keller PJ, Brooks SV, Feldman EL (2013) The role of oxidative stress in nervous system aging. PLoS One 8:e68011
Smith MA, Casadesus G, Joseph JA, Perry G (2002) Amyloid-β and τ serve antioxidant functions in the aging and Alzheimer brain. Free Radic Biol Med 33:1194–1199
Subiros N, Del Barco DG, Coro-Antich RM (2012) Erythropoietin: still on the neuroprotection road. Ther Adv Neurol Disord 5:161–173
Ugurluer G, Cebi A, Mert H, Mert N, Serin M, Erkal HS (2016) Neuroprotective effects of erythropoietin against oxidant injury following brain irradiation: an experimental study. Arch Med Sci 6:1348–1353
Uylings HB, de Brabander JM (2002) Neuronal changes in normal human aging and Alzheimer’s disease. Brain Cogn 49:268–276
Vatassery GT (1998) Vitamin E and other endogenous antioxidants in the central nervous system. Geriatrics 53(Suppl 1):S25–S27
Wang J, Sun P, Bao Y, Dou B, Song D, Li Y (2012) Vitamin E renders protection to PC12 cells against oxidative damage and apoptosis induced by single-walled carbon nanotubes. Toxicol in Vitro 26:32–41
Wang HQ, Wu HQ, Zhang XF, Liu X, Gao Z, Bu N, Sun H, Yu XR (2016) Antioxidant capacities of long-range cultured neurons enhanced by erythropoietin (EPO)-regulated Nrf2 pathway. Int J Clin Exp Pathol 9:662–672
Widl K, Brettschneider J, Schattauer D, Süssmuth S, Huber R, Ludolph AC, Tumani H (2007) Erythropoietin in cerebrospinal fluid: age-related reference values and relevance in neurological disease. Neurochem Res 32:1163–1168
Wu H, Zhao J, Chen M, Wang H, Yao Q, Fan J, Zhang M (2017) The anti-aging effect of erythropoietin via the ERK/Nrf2-ARE pathway in aging rats. J Mol Neurosci 61:449–458
Yoo SJ, Cho B, Moon C, Yu SW, Moon C (2016) Neuroprotective effects of an erythropoietin-derived peptide in PC12 cells under oxidative stress. CNS Neurol Disord Drug Targets
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 81170330).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wang, H., Fan, J., Chen, M. et al. rhEPO Enhances Cellular Anti-oxidant Capacity to Protect Long-Term Cultured Aging Primary Nerve Cells. J Mol Neurosci 62, 291–303 (2017). https://doi.org/10.1007/s12031-017-0937-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-0937-6