Abstract
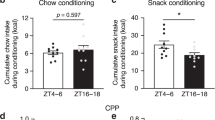
Predictable restricted feeding schedules limit food availability to a single meal at the same time each day, lead to the induction and entrainment of circadian rhythms in food-anticipatory activity, and shift daily rhythms of clock gene expression in areas of the brain that are important in the regulation of motivational and emotional state. In contrast, when food is delivered under a variable restricted feeding (VRF) schedule, at a new and unpredictable mealtime each day, circadian rhythms in food-anticipatory activity fail to develop. Here, we study the effects of VRF on the daily rhythm of plasma corticosterone and of clock gene expression in the limbic forebrain and dorsal striatum, of rats provided a 2-h access to a complete meal replacement (Ensure Plus) at an unpredictable time each day. VRF schedules varied the mealtimes within the 12 h of light (daytime VRF), the 12 h of dark (nighttime VRF), or across the 24 h light–dark cycle (anytime VRF). Our results show that contrary to the synchronizing effects of predictable restricted feeding, VRF blunts the daily corticosterone rhythm and disrupts daily rhythms of PER2 expression in a region-specific and mealtime-dependent manner.


Similar content being viewed by others
References
Ahlers I, Smajda B, Ahlersova E (1980) Circadian rhythm of plasma and adrenal corticosterone in rats: effect of restricted feeding schedules. Endocrinol Exp 14:183–190
Amir S, Lamont EW, Robinson B, Stewart J (2004) A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J Neurosci 24:781–790
Angeles-Castellanos M, Mendoza J, Escobar C (2007) Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience 144:344–355
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW (2009) Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17:2100–2102
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, Katsuura G, Makino S, Fujino MA, Kasuga M (2001) A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology 74:143–147
Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U (2008) SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134:317–328
Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, Delaunay F, Wahli W, Laudet V (2006) Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol 20:1715–1727
Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14:2950–2961
Davidson AJ, Stokkan KA, Yamazaki S, Menaker M (2002) Food-anticipatory activity and liver per1-luc activity in diabetic transgenic rats. Physiol Behav 76:21–26
Davidson AJ, Poole AS, Yamazaki S, Menaker M (2003) Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav 2:32–39
Escobar C, Martinez-Merlos MT, Angeles-Castellanos M, del Carmen MM, Buijs RM (2007) Unpredictable feeding schedules unmask a system for daily resetting of behavioural and metabolic food entrainment. Eur J Neurosci 26:2804–2814
Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ (2010) Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA 107:18664–18669
Fuller PM, Lu J, Saper CB (2008) Differential rescue of light- and food-entrainable circadian rhythms. Science 320:1074–1077
Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S (2001) Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 6:269–278
Honma KI, Honma S, Hiroshige T (1983) Critical role of food amount for prefeeding corticosterone peak in rats. Am J Physiol 245:R339–R344
Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, Stewart J, Amir S (2010) Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci 30:14046–14058
Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS (2011) Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA 108:1657–1662
Lamont EW, Robinson B, Stewart J, Amir S (2005) The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci USA 102:4180–4184
Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U (2001) Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J 20:7128–7136
Liu C, Li S, Liu T, Borjigin J, Lin JD (2007) Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 447:477–481
Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR, Sole MJ (2008) Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol 294:R1675–R1683
Mistlberger RE (1994) Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev 18:171–195
Mistlberger RE, Yamazaki S, Pendergast JS, Landry GJ, Takumi T, Nakamura W (2008) Comment on “Differential rescue of light- and food-entrainable circadian rhythms”. Science 322:675, author reply 675
Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P (2008) The NAD+−dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134:329–340
Richter CP (1922) A behavioristic study of the activity of the rat. Comp Psychol Monogr 1:1–55
Segall LA, Perrin JS, Walker CD, Stewart J, Amir S (2006) Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience 140:753–757
Segall LA, Verwey M, Amir S (2008) Timed restricted feeding restores the rhythms of expression of the clock protein, Period2, in the oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in adrenalectomized rats. Neuroscience 157:52–56
Segall LA, Milet A, Tronche F, Amir S (2009) Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice. Neurosci Lett 457:58–60
Stephan FK, Swann JM, Sisk CL (1979) Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol 25:346–363
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045
Valle FP (1981) Detrimental effects of irregular meals on rats’ ability to adjust to meal feeding. Am J Psychol 94:3–11
Verwey M, Amir S (2009) Food-entrainable circadian oscillators in the brain. Eur J Neurosci 30:1650–1657
Verwey M, Khoja Z, Stewart J, Amir S (2007) Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience 147:277–285
Verwey M, Khoja Z, Stewart J, Amir S (2008) Region-specific modulation of PER2 expression in the limbic forebrain and hypothalamus by nighttime restricted feeding in rats. Neurosci Lett 440:54–58
Verwey M, Lam GY, Amir S (2009) Circadian rhythms of PERIOD1 expression in the dorsomedial hypothalamic nucleus in the absence of entrained food-anticipatory activity rhythms in rats. Eur J Neurosci 29:2217–2222
Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S (2001) Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci 13:1190–1196
Watson RE Jr, Wiegand SJ, Clough RW, Hoffman GE (1986) Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159
Yi CX, Challet E, Pévet P, Kalsbeek A, Escobar C, Buijs RM (2008) A circulating ghrelin mimetic attenuates light-induced phase delay of mice and light-induced Fos expression in the suprachiasmatic nucleus of rats. Eur J Neurosci 27:1965–1972
Yoshida M, Kiyofuji H, Koyanagi S, Matsuo A, Fujioka T, To H, Higuchi S, Ohdo S (2006) Glucocorticoid is involved in food-entrainable rhythm of mu-opioid receptor expression in mouse brainstem and analgesic effect of morphine. J Pharmacol Sci 101:77–84
Acknowledgments
This work was supported by the Fonds de la Recherche en Santé Québec, the Canadian Institutes of Health Research, and the Concordia University Research Chairs Program. We would also like to thank Germain Y.M. Lam for his assistance with animal care.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verwey, M., Amir, S. Variable Restricted Feeding Disrupts the Daily Oscillations of Period2 Expression in the Limbic Forebrain and Dorsal Striatum in Rats. J Mol Neurosci 46, 258–264 (2012). https://doi.org/10.1007/s12031-011-9529-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9529-z