Abstract
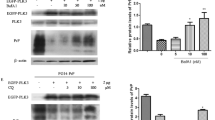
Octarepeats region sequence is one of the most important characteristics of PrP topology. To explore the mechanism of deleted and inserted octarepeats mutants PrP-caused apoptosis, wild-type PrP (PrP-PG5), and PrP with deleted octarepeats (PrP-PG0) and with four (PrP-PG9) and seven (PrP-PG12) extra octarepeats were transiently induced into SH-SY5Y cell. The results indicated PrP-PG9 and PrP-PG12 mainly retained in fraction of cytoplasm, while PrP-PG5 and PrP-PG0 presented both in cell membrane and cytoplasm. Cells expressing PrP-PG9 and PrP-PG12 were sensitive to endoplasmic reticulum (ER) stimuli, tunicamycin, and brefeldin A. ER-stress-related proteins, Grp94, XBP1, TRAF2, and CHOP, were significantly increased in cells expressing PrP-PG9 and PrP-PG12, while Grp78 increased markedly 12 h and pro-caspase-12 decreased sharply 20 h post-transfection. It indicates that expressions of PrP mutants with inserted octarepeats cause ER stress and lead to cell apoptosis lately. Meanwhile, cellular Cytochrome C increased and Bcl-2 decreased obviously in cells expressing PrP-PG0, indicating triggering a mitochondrial-related apoptosis. These data highlight that PrP mutants in region of octarepeats may undergo different pathways to trigger cell apoptosis, in which PrPs with inserted octarepeats via ER stress and PrP mutant without octarepeats via mitochondrial-related pathway.







Similar content being viewed by others
References
Aguzzi A, Polymenidou M (2004) Mammalian prion biology: one century of evolving concepts. Cell 116:313–327
An R, Dong CF, Lei YJ et al (2008) PrP mutants with different numbers of octarepeat sequences are more susceptible to the oxidative stress. Sci China C Life Sci 51:630–639
Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Faournoux P (2004) Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem 279:5288–5297
Brown DR, Besinger A (1998) Prion protein expression and superoxide dismutase activity. Biochem J 334:423–429
Brown DR, Qin K, Herms JW et al (1997) The cellular prion protein binds copper in vivo. Nature 390:684–687
Chen L, Yang Y, Han J et al (2007) Removal of the glycosylation of prion protein provokes apoptosis in SF126. J Biochem Mol Biol 41:662–669
Cherasse Y, Maurin AC, Chaveroux C et al (2007) The p300/CBP-associated factor (PCAF) is a cofactor of ATF4 for amino acid-regulated transcription of CHOP. Nucleic Acids Res 35:5954–5965
Collinge J, Palmer MS, Sidle KC et al (1995) Transmission of the familial insomnia to laboratory animals. Lancet 346:569–570
Dong CF, Shi S, Wang XF et al (2008) The N-terminus of PrP is responsible for interacting with tubulin and fCJD related PrP mutants possess stronger inhibitive effect on microtubule assembly in vitro. Arch Biochem Biophys 480:83–92
Flechsig E, Shmerling D, Hegyi L et al (2000) Prion protein devoid of the octapeptide repeat region restores susceptibility to scrapie in PrP knockout mice. Neuro 27:399–408
Goldfarb LG, Brown P, McCombie WR et al (1991) Transmissible familial Creutzfeldt-Jakob disease associated with five, seven, and eight extra octapeptide coding repeats in the PRNP gene. Proc Natl Acad Sci U S A 88:10926–10930
Harris DA (2003) Trafficking, turnover and membrane topology of PrP: protein function in prion disease. Br Med Bull 66:71–85
Hegde RS, Rane NS (2003) Prion protein trafficking and the development of neurodegeneration. Trends Neurosci 26:337–339
Hegde RS, Mastrianni JA, Scott MR et al (1998) A transmembrane form of the prion protein in neurodegenerative disease. Science 279:827–834
Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C (2003) Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J 22:5435–5445
Hornshaw MP, McDermott JR, Candy JM (1995) Copper binding to the N-terminal tandem repeat region of mammalian and avian prion protein: structural studies using synthetic peptides. Biochem Biophys Res Commun 207:621–629
Hosoi T, Hyoda K, Okuma Y, Nomura Y, Ozawa K (2007) Geldanamycin induces CHOP expression through a 4-(2-amimoethyl)-benzenesulfonyl fluoride-responsive serine protease. Cell Res 17:184–186
Hosoi T, Sasaki M, Baba S, Ozawa K (2009) Effect of pranoprofen on endoplasmic reticulum stress in the primary cultured glial cells. Neurochem Int 54:1–6
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative disease. Cell Death Differ 13:385–392
Pauly PC, Harris DA (1998) Copper stimulates endocytosis of the prion protein. J Biol Chem 273:33107–33110
Prusiner SB (1998) Prions. Proc Natl Acad Sci U S A 95:13363–13383
Prusiner SB, Groth D, Serban A et al (1993) Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc Natl Acad Sci U S A 90:10608–10612
Suzette A, Priola B, Chesebro B (1998) Abnormal properties of prion protein with insertional mutations in different cell types. J Biol Chem 273:11980–11985
Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D (1998) Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J 17:5708–5717
Wang Q, He ZZ, Zhang JH et al (2005) Overexpression of endoplasmic reticulum molecular chaperone Grp94 and Grp78 in human lung cancer tissues and its significance. Cancer Detect Prev 29:544–551
Wang XF, Guo YJ, Zhang BY et al (2007) Creutzfeldt-Jakob disease in a Chinese patient with a novel seven extra-repeat insertion in PRNP. J Neurol Neurosurg Psychiatry 78:201–203
Winklhofer KF, Tatzelt J (2006) The role of chaperones in Parkinson’s disease and prion diseases. Molecular Chaperones in Health and Diseases 172:221–258
Xuan B, Qian Z, Toriqoi E, Yu D (2009) Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J Virol 83:3463–3476
Yin SM, Yu SL, Li CY et al (2006) Prion proteins with insertion mutations have altered N-terminal conformation and increased ligand binding activity and are more susceptible to oxidative attack. J Biol Chem 281:10698–10705
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress produce a highly active transcription factor. Cell 107:881–891
Zong WX, Li C, Hatzivassiliou G (2003) Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol 162:59–69
Acknowledgments
This work was supported by Chinese National Natural Science Foundation Grants 30771914 and 30800975, National Science and Technology Task Force Project (2006BAD06A13-2), Institution Technique R&D Grant (2008EG150300), National Basic Research Program of China (973 Program) (2007CB310505), China Mega-Project for Infectious Disease (2009ZX10004-101), and the SKLID development Grant (2008SKLID102 and 2008SKLID202).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, K., Wang, X., Shi, Q. et al. Human Prion Protein Mutants with Deleted and Inserted Octarepeats Undergo Different Pathways to Trigger Cell Apoptosis. J Mol Neurosci 43, 225–234 (2011). https://doi.org/10.1007/s12031-010-9387-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-010-9387-0