Abstract
Introduction
A large body of evidence has implicated the signal transducer and activator of transcription (STAT) family and particularly the ubiquitously expressed STAT3 protein in the pathogenesis of colorectal, hepatocellular, gastric and pancreatic carcinoma.
Discussion
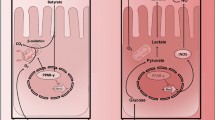
Concomitantly, an increasing body of epidemiological evidence has linked obesity and its associated pro-inflammatory state with the development of gastrointestinal cancers. Visceral adipose tissue is no longer considered inert and is known to secrete a number of adipocytokines such as leptin, interleukin (IL)-6, IL-8, IL-1β and tumour necrosis factor-alpha (TNF-α) into the surrounding environment. Interestingly, these adipocytokines are strongly linked with the Janus kinase (JAK)/STAT pathway of signal transduction and there is experimental evidence linking IL-1β, IL-8 and TNF-α to JAK/STAT signaling in other tissues. The result is an up-regulation of a wide range of anti-apoptotic, pro-metastatic and pro-angiogenic genes and processes. This is particularly relevant for gastrointestinal malignancy as these factors have the potential to signal adjacent endothelial cells in a paracrine manner.
Conclusion
This review examines the potential role of the STAT3 signaling pathway in the pathogenesis of obesity-related gastrointestinal malignancy and the potential therapeutic role of STAT3 blockade given its status as a signaling hub for a number of inflammatory adipocytokines.


Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM (2020), “GLOBOCAN 2008 v2.0. Cancer incidence and mortality worldwide: IARC CancerBase No. 10”, Lyon, France: International Agency for Research on Cancer; 2010.
Kaidar-Person O, Bar-Sela G, Person B. The two major epidemics of the twenty-first century: obesity and cancer. Obes Surg. 2011;21(11):1792–7.
Reeves GK et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134.
Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253–6.
Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nature Rev Cancer. 2004;4(2):97–105.
Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Rev Cancer. 2009;9(11):798–809.
Darnell Jr JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–21.
Stark GR et al. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–64.
Lim CP, Cao X. Structure, function, and regulation of STAT proteins. Mol BioSyst. 2006;2(11):536–50.
Mao X et al. Structural bases of unphosphorylated STAT1 association and receptor binding. Mol Cell. 2005;17(6):761–71.
Xu X, Sun YL, Hoey T. Cooperative DNA binding and sequence-selective recognition conferred by the STAT amino-terminal domain. Science. 1996;273(5276):794–7.
Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature. 1998;394(6689):145–51.
Schindler C, Strehlow I. Cytokines and STAT signaling. Adv Pharmacol. 2000;47:113–74.
Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Ann Rev Immunol. 1998;16:293–322.
Lin JX et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2(4):331–9.
Meraz MA et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84(3):431–42.
Kiu H, Nicholson SE. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. 2012;30(2):88–106.
Kisseleva T et al. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285(1–2):1–24.
Schindler C, Darnell Jr JE. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Ann Rev Biochem. 1995;64:621–51.
Starr R, Hilton DJ. Negative regulation of the JAK/STAT pathway. BioEssays: news and reviews in molecular. Cell Dev Biol. 1999;21(1):47–52.
Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282(28):20059–63.
Mantovani A et al. Cancer-related inflammation. Nature. 2008;454(7203):436–44.
Prigge JR, Schmidt EE. Interaction of protein inhibitor of activated STAT (PIAS) proteins with the TATA-binding protein, TBP. J Biol Chem. 2006;281(18):12260–9.
Chung CD et al. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278(5344):1803–5.
Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer—more than a “gut” feeling? Cell Div. 2010;5:14.
Wegenka UM et al. Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol. 1993;13(1):276–88.
Zhong Z, Wen Z, Darnell Jr JE. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264(5155):95–8.
Cheon H, Yang J, Stark GR. The functions of signal transducers and activators of transcriptions 1 and 3 as cytokine-inducible proteins. J Interferon Cytokine Res. 2011;31(1):33–40.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45.
Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21(1):11–9.
Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7(1):41–51.
Lee H et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15(4):283–93.
Ogura H et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29(4):628–36.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Ernst M, Putoczki TL. Stat3: linking inflammation to (gastrointestinal) tumourigenesis. Clin Exp Pharmacol Physiol. 2012;39(8):711–8.
Turkson J et al. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18(5):2545–52.
Caldenhoven E et al. STAT3beta, a splice variant of transcription factor STAT3, is a dominant negative regulator of transcription. J Biol Chem. 1996;271(22):13221–7.
Bromberg JF et al. Stat3 as an oncogene. Cell. 1999;98(3):295–303.
Niu G et al. Gene therapy with dominant-negative Stat3 suppresses growth of the murine melanoma B16 tumor in vivo. Cancer Res. 1999;59(20):5059–63.
Catlett-Falcone R et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10(1):105–15.
Chai SK, Nichols GL, Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol. 1997;159(10):4720–8.
Fernandes A, Hamburger AW, Gerwin BI. ErbB-2 kinase is required for constitutive stat 3 activation in malignant human lung epithelial cells. Int J Cancer. 1999;83(4):564–70.
Garcia R et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast a. carcinoma cells. Oncogene. 2001;20(20):2499–513.
Grandis JR et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest. 1998;102(7):1385–92.
Akira S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene. 2000;19(21):2607–11.
Cai L et al. Growth inhibition of human ovarian cancer cells by blocking STAT3 activation with small interfering RNA. Eur J Obstet Gynecol Reprod Biol. 2010;148(1):73–80.
Gao L et al. Down-regulation of signal transducer and activator of transcription 3 expression using vector-based small interfering RNAs suppresses growth of human prostate tumor in vivo. Clin Cancer Res. 2005;11(17):6333–41.
Gao L et al. Inhibition of STAT3 and ErbB2 suppresses tumor growth, enhances radiosensitivity, and induces mitochondria-dependent apoptosis in glioma cells. Int J Radiat Oncol Biol Phys. 2010;77(4):1223–31.
Li X et al. STAT3 blockade with shRNA enhances radiosensitivity in Hep-2 human laryngeal squamous carcinoma cells. Oncol Rep. 2010;23(2):345–53.
Lysaght J et al. T lymphocyte activation in visceral adipose tissue of patients with oesophageal adenocarcinoma. Brit J Surg. 2011;98(7):964–74.
Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116(1):33–5.
Donohoe CL et al. Obesity and gastrointestinal cancer. Brit J Surg. 2010;97(5):628–42.
Margetic S et al. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26(11):1407–33.
Lysaght J et al. Pro-inflammatory and tumour proliferative properties of excess visceral adipose tissue. Cancer Lett. 2011;312(1):62–72.
Baumann H et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93(16):8374–8.
Morton NM et al. Leptin action in intestinal cells. J Biol Chem. 1998;273(40):26194–201.
Barrenetxe J et al. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut. 2002;50(6):797–802.
Dalwadi H et al. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res. 2005;11(21):7674–82.
Francois F et al. The association of gastric leptin with oesophageal inflammation and metaplasia. Gut. 2008;57(1):16–24.
Howard JM, Pidgeon GP, Reynolds JV. Leptin and gastro-intestinal malignancies. Obes Rev. 2010;11(12):863–74.
Kieffer TJ, Heller RS, Habener JF. Leptin receptors expressed on pancreatic beta-cells. Biochem Biophys Res Commun. 1996;224(2):522–7.
Mix H et al. Expression of leptin and leptin receptor isoforms in the human stomach. Gut. 2000;47(4):481–6.
Morton NM et al. Leptin signalling in pancreatic islets and clonal insulin-secreting cells. J Mol Endocrinol. 1999;22(2):173–84.
Uchiyama T et al. Leptin receptor is involved in STAT3 activation in human colorectal adenoma. Cancer Sci. 2011;102(2):367–72.
Uchiyama T et al. Role of the long form leptin receptor and of the STAT3 signaling pathway in colorectal cancer progression. Int J Oncol. 2011;39(4):935–40.
Saxena NK et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67(6):2497–507.
Pai R et al. Leptin activates STAT and ERK2 pathways and induces gastric cancer cell proliferation. Biochem Biophys Res Commun. 2005;331(4):984–92.
Aparicio T et al. Leptin stimulates the proliferation of human colon cancer cells in vitro but does not promote the growth of colon cancer xenografts in nude mice or intestinal tumorigenesis in Apc(Min/+) mice. Gut. 2005;54(8):1136–45.
Hardwick JC et al. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121(1):79–90.
Hoda MR et al. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Brit J Surg. 2007;94(3):346–54.
Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38.
Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30(9):1005–14.
Heinrich PC et al. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314.
Shirota K et al. Interleukin-6 and its receptor are expressed in human intestinal epithelial cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;58(4):303–8.
Schneider MR et al. Interleukin-6 stimulates clonogenic growth of primary and metastatic human colon carcinoma cells. Cancer Lett. 2000;151(1):31–8.
Leu CM et al. Interleukin-6 acts as an antiapoptotic factor in human esophageal carcinoma cells through the activation of both STAT3 and mitogen-activated protein kinase pathways. Oncogene. 2003;22(49):7809–18.
Block KM et al. IL-6 stimulates STAT3 and Pim-1 kinase in pancreatic cancer cell lines. Pancreas. 2012;41(5):773–81.
Okitsu K et al. Involvement of interleukin-6 and androgen receptor signaling in pancreatic cancer. Gene Chromosome Cancer. 2010;1(8):859–67.
Lesina M et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–69.
Liu H et al. Molecular dissection of human oncostatin M-mediated signal transductions through site-directed mutagenesis. Int J Mol Med. 2009;23(2):161–72.
Hermanns HM, Radtke S, Haan C, Tavernier J, Heinrich PC, et al. Contributions of leukemia inhibitory factor receptor and oncostatin M receptor to signal transduction in heterodimeric complexes with glycoprotein 130. J Immunol. 1999;163(12):6651–8.
Stephens JM, Lumpkin SJ, Fishman JB. Activation of signal transducers and activators of transcription 1 and 3 by leukemia inhibitory factor, oncostatin-M, and interferon-gamma in adipocytes. J Biol Chem. 1998;273(47):31408–16.
Migita K et al. CP690,550 inhibits oncostatin M-induced JAK/STAT signaling pathway in rheumatoid synoviocytes. Arthritis Res Ther. 2011;13(3):R72.
Balhoff JP, Stephens JM. Highly specific and quantitative activation of STATs in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1998;247(3):894–900.
Lin SK et al. MEK/ERK and signal transducer and activator of transcription signaling pathways modulate oncostatin M-stimulated CCL2 expression in human osteoblasts through a common transcription factor. Arthritis Rheum. 2004;50(3):785–93.
Kok SH et al. Oncostatin M-induced CCL2 transcription in osteoblastic cells is mediated by multiple levels of STAT-1 and STAT-3 signaling: an implication for the pathogenesis of arthritis. Arthritis Rheum. 2009;60(5):1451–62.
Fossey SL et al. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer. 2011;11:125.
Li WQ, Dehnade F, Zafarullah M. Oncostatin M-induced matrix metalloproteinase and tissue inhibitor of metalloproteinase-3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathway. J Immunol. 2001;166(5):3491–8.
Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–147.
Park JI et al. Interleukin-1beta can mediate growth arrest and differentiation via the leukemia inhibitory factor/JAK/STAT pathway in medullary thyroid carcinoma cells. Cytokine. 2005;29(3):125–34.
Samavati L et al. STAT3 tyrosine phosphorylation is critical for interleukin 1 beta and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol Immunol. 2009;46(8–9):1867–77.
Shankar E et al. High-fat diet activates pro-inflammatory response in the prostate through association of Stat-3 and NF-kappaB. Prostate. 2012;72(3):233–43.
Biswas SK, Sodhi A. Tyrosine phosphorylation-mediated signal transduction in MCP-1-induced macrophage activation: role for receptor dimerization, focal adhesion protein complex and JAK/STAT pathway. Int Immunopharmacol. 2002;2(8):1095–107.
Biswas P et al. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91(1):258–65.
Jougasaki M et al. Statins suppress interleukin-6-induced monocyte chemo-attractant protein-1 by inhibiting Janus kinase/signal transducers and activators of transcription pathways in human vascular endothelial cells. Brit J Pharmacol. 2010;159(6):1294–303.
Pitroda SP et al. Tumor endothelial inflammation predicts clinical outcome in diverse human cancers. PloS One. 2012;7(10):e46104.
Miscia S et al. Tumor necrosis factor alpha (TNF-alpha) activates Jak1/Stat3-Stat5B signaling through TNFR-1 in human B cells. Cell Growth Differ. 2002;13(1):13–8.
Loetscher H et al. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990;61(2):351–9.
Liu Z et al. Diet-induced obesity elevates colonic TNF-alpha in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem. 2012;23(10):1207–13.
Jain SS, AshokKumar M, Bird RP. Differential expression of TNF-alpha signaling molecules and ERK1 in distal and proximal colonic tumors associated with obesity. Tumour Biol. 2011;32(5):1005–12.
Ning Y et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128(9):2038–49.
Kim CS et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes. 2006;30(9):1347–55.
Bruun JM et al. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab. 2004;286(1):E8–E13.
Abolhassani M et al. Leptin receptor-related immune response in colorectal tumors: the role of colonocytes and interleukin-8. Cancer Res. 2008;68(22):9423–32.
Cacev T et al. Influence of interleukin-8 and interleukin-10 on sporadic colon cancer development and progression. Carcinogenesis. 2008;29(8):1572–80.
Wilson AJ, Byron K, Gibson PR. Interleukin-8 stimulates the migration of human colonic epithelial cells in vitro. Clin Sci. 1999;97(3):385–90.
Shi Q et al. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res. 1999;5(11):3711–21.
Hussain F et al. The expression of IL-8 and IL-8 receptors in pancreatic adenocarcinomas and pancreatic neuroendocrine tumours. Cytokine. 2010;49(2):134–40.
Kitadai Y et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Clin Pathol. 1998;152(1):93–100.
Jenkins GJ et al. Immunohistochemical study of nuclear factor-kappaB activity and interleukin-8 abundance in oesophageal adenocarcinoma; a useful strategy for monitoring these biomarkers. Am J Clin Pathol. 2007;60(11):1232–7.
Fitzgerald RC et al. Inflammatory gradient in Barrett's oesophagus: implications for disease complications. Gut. 2002;51(3):316–22.
Trevino JG et al. Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res. 2005;65(16):7214–22.
Gharavi NM et al. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J Biol Chem. 2007;282(43):31460–8.
Burger M et al. KSHV-GPCR and CXCR2 transforming capacity and angiogenic responses are mediated through a JAK2-STAT3-dependent pathway. Oncogene. 2005;24(12):2067–75.
Oka M et al. Signal transducer and activator of transcription 3 upregulates interleukin-8 expression at the level of transcription in human melanoma cells. Clin Exp Dermatol. 2010;19(8):e50–5.
Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–41.
Starr R, Hilton DJ. Negative regulation of the JAK/STAT pathway. BioEssays. 1999;21(1):47–52.
Turkson J et al. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276(48):45443–55.
Page BD, Ball DP, Gunning PT. Signal transducer and activator of transcription 3 inhibitors: a patent review. Expert Opin Ther Pat. 2011;21(1):65–83.
Ren Z et al. Identification of a high-affinity phosphopeptide inhibitor of Stat3. Bioorg Med Chem Lett. 2003;13(4):633–6.
Song H et al. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A. 2005;102(13):4700–5.
Xu X et al. Chemical probes that competitively and selectively inhibit Stat3 activation. PLoS One. 2009;4(3):e4783.
Berg T. Inhibition of transcription factors with small organic molecules. Curr Opin Chem Biol. 2008;12(4):464–71.
Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Invest Drugs. 2009;18(1):45–56.
Gunning PT et al. Targeting protein-protein interactions: suppression of Stat3 dimerization with rationally designed small-molecule, nonpeptidic SH2 domain binders. ChemBioChem. 2008;9(17):2800–3.
Turkson J et al. Inhibition of constitutive signal transducer and activator of transcription 3 activation by novel platinum complexes with potent antitumor activity. Mol Cancer Ther. 2004;3(12):1533–42.
Zhang X et al. A novel small-molecule disrupts Stat3 SH2 domain-phosphotyrosine interactions and Stat3-dependent tumor processes. Biochem Pharmacol. 2010;79(10):1398–409.
University of Pittsburgh. STAT3 decoy in head and neck cancer. NCT00696176.
Leong PL et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci U S A. 2003;100(7):4138–43.
Sen M et al. Lack of toxicity of a STAT3 decoy oligonucleotide. Cancer Chemother Pharmacol. 2009;63(6):983–95.
M.D. Anderson Cancer Center (2012) STAT3 inhibitor for solid tumors. Accessed: 1 Oct 2012.
Wang S, Chen J, Zhao Y, et al. (2010) Stat3 inhibitors and therapeutic methods using the same. (WO10077589).
Chen J et al. Structure-based design of conformationally constrained, cell-permeable STAT3 inhibitors. ACS Med Chem Lett. 2010;1(2):85–9.
Jones G et al. Development and validation of a genetic algorithm for flexible docking. J Mol Biolf. 1997;267(3):727–48.
Sebti SM, Hamilton AD, Turkson J, et al. (2008) Use of SH2 Stat3/Stat1 peptidomimetics as anticancer drugs. (WO08070833).
Michejda, C. J., Tarasova NI, Timofeeva O, et al. (2008) Peptide-based STAT inhibitor. (WO08151037).
Timofeeva OA et al. Rationally designed inhibitors identify STAT3 N-domain as a promising anticancer drug target. ACS Chem Biol. 2007;2(12):799–809.
Funding
K.E. O’Sullivan is funded by a scholarship grant from the Irish Cancer Society (grant code CRS120SU).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Sullivan, K.E., Reynolds, J.V., O’Hanlon, C. et al. Could Signal Transducer and Activator of Transcription 3 be a Therapeutic Target in Obesity-Related Gastrointestinal Malignancy?. J Gastrointest Canc 45, 1–11 (2014). https://doi.org/10.1007/s12029-013-9555-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-013-9555-x