Abstract
Background
After traumatic brain injury (TBI), fever is frequent. Brain temperature (BT), which is directly linked to body temperature, may influence brain physiology. Increased body and/or BT may cause secondary brain damage, with deleterious effects on intracranial pressure (ICP), cerebral perfusion pressure (CPP), and outcome.
Methods
Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI), a prospective multicenter longitudinal study on TBI in Europe and Israel, includes a high resolution cohort of patients with data sampled at a high frequency (from 100 to 500 Hz). In this study, simultaneous BT, ICP, and CPP recordings were investigated. A mixed-effects linear model was used to examine the association between different BT levels and ICP. We additionally focused on changes in ICP and CPP during the episodes of BT changes (Δ BT ≥ 0.5 °C lasting from 15 min to 3 h) up or downward. The significance of ICP and CPP variations was estimated with the paired samples Wilcoxon test (also known as Wilcoxon signed-rank test).
Results
Twenty-one patients with 2,435 h of simultaneous BT and ICP monitoring were studied. All patients reached a BT of 38 °C and experienced at least one episode of ICP above 20 mm Hg. The linear mixed-effects model revealed an association between BT above 37.5 °C and higher ICP levels that was not confirmed for lower BT. We identified 149 episodes of BT changes. During BT elevations (n = 79) ICP increased, whereas CPP was reduced; opposite ICP and CPP variations occurred during episodes of BT reduction (n = 70). All these changes were of moderate clinical relevance (increase of ICP of 4.5 and CPP decrease of 7.5 mm Hg for BT rise, and ICP reduction of 1.7 and CPP elevation of 3.7 mm Hg during BT defervescence), even if statistically significant (p < 0.0001). It has to be noted, however, that a number of therapeutic interventions against intracranial hypertension was documented during those episodes.
Conclusions
Patients after TBI usually develop BT > 38 °C soon after the injury. BT may influence brain physiology, as reflected by ICP and CPP. An association between BT exceeding 37.5 °C and a higher ICP was identified but not confirmed for lower BT ranges. The relationship between BT, ICP, and CPP become clearer during rapid temperature changes. During episodes of temperature elevation, BT seems to have a significant impact on ICP and CPP.
Similar content being viewed by others
Introduction
The injured brain is extremely sensitive and vulnerable to body temperature changes [1, 2]. An increase in temperature leads to an increase in cerebral metabolism, with augmented cerebral blood flow, and a concurrent increase in cerebral blood volume. If the compensatory mechanisms are exhausted, this high cerebral blood volume [3] may raise ICP [4]. In an experimental model of brain contusion, hyperthermia (39 °C for 3 h) caused enlargement of the contusion volume and had a negative effect on outcome [5].
High body temperature can also worsen the cerebral ischemia. In experimental models of brain ischemia, hyperthermia increased the release of glutamate [6] and the extent of tissue damage [7]. Even though TBI is a different pathology from acute ischemic stroke, there is evidence [8] that abnormalities in flow metabolism coupling and areas of true ischemia are fairly common in patients with TBI.
Most patients with moderate and severe TBI experience hyperthermia during their intensive care unit (ICU) stay [9,10,11] and are therefore exposed to the deleterious effects of increased temperature on ICP, brain metabolism, and risk of ischemia.
A substantial proportion of experimental and clinical evidence on the interplay between hyperthermia and the brain is based on temperature measured outside the brain, either with sensors measuring temperature in the bladder [12,13,14], rectum [12, 13, 15,16,17], esophagus [12], pulmonary artery [13, 18], and jugular vein [15], which are collectively indicated as core temperature (CT), or placed externally (axillary [13] and tympanic [12] temperatures).
Unfortunately, temperatures measured outside the brain may markedly underestimate the BT, especially when it rises [18]. The mean difference between BT and CT is 0.3–0.4 °C, but it may be significantly higher during the development of pyrexia (up to 2.6 °C) depending on several factors [15, 18,19,20,21]. Nevertheless, direct BT monitoring in patients with brain injuries is rarely used [22].
We consulted a centralized data collection covering several centers in Europe [23] to obtain information on simultaneously monitored BT and ICP. The aims of this study were the following:
-
1.
To provide a general description of BT, ICP, and CPP in a limited sample of patients with TBI.
-
2.
To clarify the relationships between BT, ICP, and CPP during acute BT changes.
Methods
Patient Population
Of the 2,138 patients in the ICU in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) data collection, a subgroup of 277 patients had high frequency digital signals from ICU monitoring (full waveform resolution at sampling frequencies at least 100 Hz, provided by the patient monitors) and was named High Resolution (HR) CENTER-TBI substudy (HR CENTER-TBI). These patients were enrolled in 21 centers from January 2015 to December 2017 and treated in accordance with current evidence-based guidelines for TBI [24, 25]. In total, 102 patients from this cohort had simultaneous temperature and ICP monitoring; BT was monitored in 22 of them. Hypothermia was induced in one patient and continued throughout the whole HR monitoring. Our analysis, therefore, includes 21 patients in whom ICP was measured through parenchymal probes.
Data collection in the CENTER-TBI study adhered to ethical standards; medical ethical committees of all participating centers approved the study. Informed consent was obtained in accordance with local regulations [23].
Data Collection and Processing
Data were collected using ICM + software (Cambridge Enterprise Ltd, Cambridge, UK, http://icmplus.neurosurg.cam.ac.uk), Moberg CNS Monitor (Moberg Research Inc, Ambler, PA, https://www.moberg.com), or both. Arterial blood pressure (ABP) was obtained through arterial lines connected to pressure transducers. ICP was acquired from an intraparenchymal strain gauge probe (Codman ICP MicroSensor; Codman & Shurtleff Inc, Raynham, MA) or parenchymal fiber optic pressure sensor (Camino ICP Monitor; Integra Life Sciences, Plainsboro, NJ, https://www.integralife.com/). BT was measured through invasive parenchymal monitoring (Licox probe; Integra, Licox Brain Oxygen Monitoring System, Plainboro, NJ), usually placed in the frontal lobe. Signal processing was done with ICM + software. Signal artifacts were removed partially automatically and partially manually. The whole process of HR CENTER-TBI signal acquisition and data processing is described in previous publications [26, 27]. Because none of the included patients had an induced hypothermia, BT lower than 36.0 °C was excluded from the final analysis.
Parallel to the digital HR monitoring, information on specific therapeutic interventions (such as osmotherapy, changes in sedation, suctioning, etc.) was recorded and synchronized to the corresponding monitored variables using ICM + . Interventions and their timing were subsequently extracted using HDFView Software (The HDF5 Group, https://www.hdfgroup.org) and analyzed.
CT was not recorded at high frequencies in the selected patient cohort, and thus the information on maximal daily CT (with the probe located in rectum, bladder, esophagus, tympanum, and nasopharynx) together with the epidemiological data were accessed using a bespoke data management tool, Neurobot (http://neurobot.incf.org) developed by the International Neuroinformatics Coordinating Facility (INCF; www.incf.org), vs. 2.1. Analysis was done using the files available from July 2019.
Statistical Analysis
Data are summarized as mean and standard deviation or as median and interquartile range (IQR). For the general description of BT, ICP, and CPP, colored maps were plotted (with per-minute data presented on a color scale) for the first 7 days of HR monitoring. Only simultaneous recordings of ICP and BT were analyzed, and missing values were excluded from the analysis.
CT was recorded daily by the investigators as the maximum and lowest temperature measured during a 24-h interval. To provide a comparison between BT and CT, maximum daily CT was compared with the highest BT recorded during the corresponding monitoring day.
To examine the relations between absolute values of six BT ranges (< 36.4, 36.5–36.9, 37–37.4, 37.5–37.9, 38–38.4, and > 38.5 °C) and ICP, we used a generalized mixed-effects linear model, with a random intercept per patient (that accounts for the repeated measurements in single patients). For the model, the per-minute values of BT and ICP were used. To correct for potential confounders, the mixed model was adjusted for TBI severity using poststabilization motor Glasgow Coma Scale ratings and pupil response [28]. For every BT group, the previous BT level was used as a reference group. p values were extracted to determine the significance of differences between ICP and BT groups. The figure was plotted using BT < 36.4 as the reference group.
To assess the effects on ICP and CPP of BT changes over time, episodes of BT elevation and reduction were manually selected according to the following criteria: elevations or reductions of at least 0.5 °C lasting from 15 min to 3 h. A maximum of five episodes was identified in each patient. BT elevation and reduction episodes were analyzed separately.
To assess the significance of ICP and CPP changes in response to BT, the baseline and end episode ICP and CPP within an episode were compared using the paired samples Wilcoxon test (also known as Wilcoxon signed-rank test). A p value < 0.05 was considered significant. ICP more than 20 mm Hg was defined as high ICP (hICP), and CPP below 60 mm Hg was used as an indicator of low cerebral perfusion. To define potentially harmfully high BT level, we used a threshold of BT of 38 °C.
All statistical analysis was done using R (“R: A language and environment for statistical computing.” R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/).
Results
Patients’ Characteristics and Monitoring
The study comprised 21 patients from four centers with simultaneous BT and ICP HR monitoring. Details of their baseline characteristics are given in Table 1. There were 18 men, with a median age of 50 years (IQR 36–55). Monitoring was established within 2 days after ICU admission; a total of 3,483 monitoring hours (median per patient 123; IQR 84–214 h) were analyzed. The final analysis included 2,435 h of monitoring (after excluding missing values) with simultaneous ICP and BT measurements.
Twenty of 21 patients had daily maximum CTs recorded, for a total of 93 ICU days during which both BT and CT were measured.
BT, ICP, and CPP
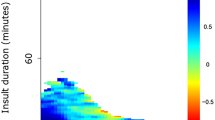
The median BT for all the patients was 37.6 °C (IQR 37.3–37.9), with the lowest value of 36.0 °C reached in five patients and the highest, 39.7 °C, reached in one. All patients reached a BT of 38 °C or higher during the monitoring. BT varied widely among the patients. Figure 1a presents the BT color map during the first week of monitoring. BT maximum daily values of 38 °C or higher were observed during 65 days of matched (BT and CT) monitoring (70%). CT maximum daily temperatures exceeded that threshold only in 46 days of matched recording (49%) (Fig. 2).
Comparison between maximal daily BT with the corresponding maximal CT. The image represents 93 available comparisons of maximal daily BT (from HR data) with the corresponding daily maximal CT (recorded manually) in 20 patients. BT and CT dots are connected by the line but, in fact, represent single measurements. In the majority of cases, BT (blue dots/line) are higher than CT (red dots/line) (n = 63, 68%). Maximal difference between BT and CT ranged from − 1.5 to 2 °C. BT, brain temperature, CT, core temperature, HR, high resolution, Max, maximal
Median ICP was 13 mm Hg (IQR 11–20). During approximately a third of monitoring time (31%), ICP reached > 20 mm Hg. All patients experienced at least one episode of hICP (Fig. 1b). Median of mean ABP (mABP) was 93 mm Hg (IQR 87–104). Median CPP was 83 mm Hg (IQR 79–86). All patients suffered episodes of CPP < 60 mm Hg (Fig. 1c); these episodes were short lasting, with only 5% of CPP recording time under the threshold.
The linear mixed-effects model examined the interplay between six ranges of BT and ICP (Fig. 3). BT above 37.5 °C was associated with significantly higher ICP (p < 0.001). For lower BT ranges (< 36.4 °C and 36.5–36.9 °C), this association was not confirmed.
Generalized linear mixed model effects on ICP of six BT ranges. Generalized linear mixed model, including ICP (mm Hg) as dependent variable (predicted values with 95% confidence interval) in six ranges of BT (°C) as independent variable. BT < 36.4 °C was taken as the reference group. The gray area indicates the values below the physiological BT range, which are likely to depend on active treatment. The asterisks indicate the following p values: X, < 1; * < 0.01; ** < 0.001; and *** < 0.0001. BT, brain temperature, ICP, intracranial pressure
BT Elevation/Reduction Analysis
We identified 149 episodes of BT changes (at least 0.5 °C): 79 elevations and 70 reductions (Fig. 4). The total duration of all the selected episodes was 321 h (13% of HR monitoring included in the current analysis).
Figure 5 describes ICP patterns during BT elevation (panel a) or reduction (panel b). The median BT elevation was 0.67 °C (IQR 0.57–0.9) and the median ΔICP was 4.5 mm Hg (IQR 0.7–7.1) (p < 0.0001). BT reached > 38 °C in 40 episodes (51%), and hICP was observed in 33 cases (42%). During these BT increases, mABP remained constant, and consequently CPP decreased, with a median difference of 7.5 mm Hg (IQR 0.9–13.6); this change was significant (p < 0.0001). During 44 episodes of BT elevation, 128 active therapeutic interventions were recorded (Table 2).
ICP response to BT changes. ICP at the beginning and end of BT episodes. a ICP during all the BT elevation episodes. b ICP during all the BT reduction episodes. Colored points represent different patients; all measurements in the same patient are the same color. p values for the paired samples Wilcoxon test (also known as Wilcoxon signed-rank test). BT, brain temperature, ICP, intracranial pressure
Starting from a median baseline BT of 38 °C (IQR 37.7–38.4) in the 70 episodes of BT reduction, there was a median decrease of 0.67 °C (IQR 0.58–0.81). ICP decreased as well, with a median reduction of 1.7 mm Hg (IQR − 1.22 to 6.03) (p < 0.001) (Fig. 5). Mean ABP remained constant and was accompanied by a significant (p < 0.005) but not clinically relevant increase of CPP (ΔCPP 3.7 mm Hg; IQR 1.96–9.8). During 41 episodes of BT reduction, 106 interventions were recorded (Table 2).
Discussion
Fever is frequent after traumatic, ischemic, and hemorrhagic injuries [10, 29,30,31,32]. In experimental models induced hyperthermia increases the extent of the contusion volume and is associated with a worse outcome [5]. However, spontaneous fever is different from the external heating of animal heads used in the experimental setting. Moreover, the direct effect of fever on the extent of neurological injury in clinical practice is particularly difficult to separate from the impact of infection (which very often is the cause of fever and originates complex inflammatory cascades).
However, there is some indirect evidence that spontaneous fever may precipitate neurologic injury in patients with ischemic stroke and multiple sclerosis [33,34,35]. In subarachnoid hemorrhage, fever control is associated with reduced cerebral metabolic distress, irrespective of ICP [30]. Meanwhile, in patients with TBI, there is an association between duration and intensity of fever with worse outcome [10].
Finally, temperature elevations may lead to ICP perturbations [18] and, conversely, fever treatments (both physical and pharmacological) may reduce ICP [36, 37].
Most studies have monitored body temperature by either external or internal probes. However, existing evidence demonstrates, that BT can not necessarily be predicted from systemic temperature [38]; thus, to understand brain physiology better, direct information on BT would be of great interest, even if it requires an invasive approach [39].
To our knowledge, this is the first study to use continuous, high frequency, simultaneous monitoring of BT and ICP in patients with TBI.
The first aim was to describe the behavior of BT, ICP, and CPP and their interactions in a selected sample of patients during the first week after injury. BT showed a range of changes (Fig. 1a). All patients experienced BT higher than 38 °C, which is consistent with previous data [9, 18, 21].
Intermittent daily CT recording provided, as expected, less information on the occurrence of elevated temperatures than the more granular documentation offered by HR. According to CT maximal daily values, temperatures above 38 °C were disclosed for 46 days and a pathological BT was measured for 65 days. This finding indicate that CT may underestimate the severity of hyperthermia, as reported previously [18].
Concomitantly, ICP was generally well controlled, as reflected by a median of 13 mm Hg (IQR 11–20). However, ICP fluctuated, so each patient suffered some hICP episodes and low CPP (< 60 mm Hg).
The generalized linear mixed model gave a biphasic pattern, tending toward higher ICP with BT above 37.5 °C and the opposite below BT of 37.4 °C (Fig. 3). One possible explanation is that BT ≤ 37.4 °C depends on active manipulations, generally those used to control pathologically high ICP. Even though the physiological range of BT has not been established yet, current evidence suggests that it should be higher than 37 °C [15,16,17,18,19,20,21], the normal body temperature [40]. In fact, BT was generally higher than body temperature in all papers [15,16,17,18,19,20,21] but two [14, 29]. BT below this level is not physiological and may well depend on active treatment (i.e., intentional moderate cooling) or be a side effect of other therapies (deep sedation, myorelaxants, etc.) generally used to treat hICP. Bearing these considerations in mind, we separated Fig. 3 into two areas, showing in gray the part where we suspect the effect of treatment.
Focusing on the ICP increase with BT more than 37.5 °C, our data partially contrast with some previous reports. Four studies [18, 19, 21, 41] found no consistent relationship between BT and ICP when monitoring data were pooled and analyzed. However, the pattern we describe was found in one report of 72 patients, and in another series in which ICP was studied as a function of body rather than BT [11, 17].
The second aim of this study was to elucidate the impact of rapid BT changes (from 15 min to 3 h) on ICP and CPP. We explored 149 episodes of significant BT changes (more than 0.5 °C) and found that both ICP and CPP deteriorated when BT rose. ICP and CPP changes were significant (p < 0.0001) but, more importantly, they were clinically relevant, with a median ICP increase of 4.5 mm Hg, that, in 40% of the episodes has crossed the threshold of 20 mm Hg by the end of BT elevation. During these episodes active treatment for intracranial hypertension was provided, including osmotherapy and sedative and vasoactive drugs, documented by the total of 128 interventions during 44 BT elevation episodes (Table 2). It is therefore plausible to consider that therapy mitigated the actual ICP and CPP deteriorations caused by the rise in BT. In the absence of treatment, more severe ICP and CPP alterations could well result from BT increases.
Reductions of BT were studied in 70 episodes. These events too affected ICP and CPP, reducing them both slightly but significantly.
Three previous studies looked into the relationship between ICP and rapid BT changes. Two studies from our group [9, 18] suggested an association, but this was not confirmed by Hushak et al. [21]. In clinical practice, it is a common observation at the bedside that ICP can worsen during the development of fever and a recent consensus statement on TBI management suggested the correction of hyperthermia as one of the first steps for ICP control [42].
Our analysis has limitations: first, it involved a limited number of patients in few centers. Generalization of our results, therefore, would call for a larger cohort. Second, the physiopathological hypothesis linking BT to ICP and CPP is based on changes in cerebral metabolism, blood flow and content, as suggested in the Introduction. Since we did not measure these variables, our interpretation of the findings has to be considered speculative. Moreover, our study lacks the data on temperature treatments; this makes the conclusion about the natural physiological behavior of BT and ICP more complicated. Finally, our data set did not include simultaneous and continuous high frequency recording of CT and BT, which could be extremely informative; consequently, our comparison of CT and BT was restricted to a limited data set. The graphical comparison of BT and CT (Fig. 2) have some inaccuracy, and measurements of BT and CT might not correspond perfectly.
Conclusions
BT can be monitored in patients with TBI during their time in the ICU, and temperature tends to vary widely, with frequent significant BT elevations (BT > 38 °C). A general analysis indicates that BT exceeding 37.5 °C seems to involve a concomitant rise in ICP. The relationships between BT, ICP, and CPP become clearer during rapid temperature changes. During episodes of temperature increase, BT seems to have a significant impact on ICP and CPP despite active treatment to prevent intracranial hypertension. A similar but less severe impact is seen when temperature decreases.
Abbreviations
- ICU:
-
Intensive care unit
- HR:
-
High resolution
- TBI:
-
Traumatic brain injury
- ABP:
-
Arterial blood pressure
- mABP:
-
Mean arterial blood pressure
- ICP:
-
Intracranial pressure
- CPP:
-
Cerebral perfusion pressure
- BT:
-
Brain temperature
- GSD:
-
Standard deviation
- IQR:
-
Interquartile range
- hICP:
-
High ICP
- GCS:
-
Glasgow coma scale
- GOSe:
-
Glasgow outcome scale extended
- ER:
-
Emergency room
References
Lanier W. Cerebral metabolic rate and hypothermia: their relationship with ischemic neurologic injury. J Neurosurg Anesthesiol. 1995;7(3):216–21.
Rosomoff H, Holaday D. Cerebral blood flow and cerebral oxygen consumption during hypothermia. Am J Physiol. 1954;79(1):85–8.
Heldt T, Zoerle T, Teichmann D, et al. Intracranial pressure and intracranial elastance monitoring in neurocritical care. Ann Rev Biomed Eng. 2019;21:523–49.
Cairns C, Andrews P. Management of hyperthermia in traumatic brain injury. Curr Opin Crit Care. 2002;8(2):106–10.
Dietrich W, Alonso O, Halley M, et al. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38(3):533–41.
Takagi K, Ginsberg M, Globus M, et al. Effect of hyperthermia on glutamate release in ischemic penumbra after middle cerebral artery occlusion in rats. Am J Physiol. 1994;267(5 Pt 2):1770–6.
Kim Y, Busto R, Dietrich W, et al. Delayed post-ischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke. 1996;27(12):2274–81.
Launey Y, Fryer T, Hong Y, et al. Spatial and temporal pattern of ischemia and abnormal vascular function following traumatic brain injury. JAMA Neurol. 2020;77(3):339–49.
Stocchetti N, Protti A, Lattuada M, et al. Impact of pyrexia on neurochemistry and cerebral oxygenation after acute brain injury. J Neurol Neurosurg Psychiatry. 2005;76(8):1135–9.
Greer D, Funk S, Reaven N, et al. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008;39(11):3029–35.
Nyholm L, Howells T, Lewén A, et al. The influence of hyperthermia on intracranial pressure, cerebral oximetry and cerebral metabolism in traumatic brain injury. Ups J Med Sci. 2017;122(3):177–84.
Cork R, Vaughan R, Humphrey L. Precision and accuracy of intraoperative temperature monitoring. Anesth Analg. 1983;62(2):211–4.
Stocchetti N, Rossi S, Zanier E, et al. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002;28(11):1555–62.
Nakagawa K, Hills N, Kamel H, et al. The effect of decompressive hemicraniectomy on brain temperature after severe brain injury. Neurocrit Care. 2011;15(1):101–6.
Rumana C, Gopinath S, Uzura M, et al. Brain temperature exceeds systemic temperature in head-injured patients. Crit Care Med. 1998;26(3):562–7.
Soukup J, Zauner A, Doppenberg E, et al. Relationship between brain temperature, brain chemistry and oxygen delivery after severe human head injury: the effect of mild hypothermia. Neurol Res. 2002;24(2):161–8.
Spiotta A, Stiefel M, Heuer G, et al. Brain hyperthermia after traumatic brain injury does not reduce brain oxygen. Neurosurgery. 2008;62(4):864–72.
Rossi S, Zanier E, Mauri I, et al. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry. 2001;71(4):448–54.
Verlooy J, Heytens L, Veeckmans G, et al. Intracerebral temperature monitoring in severely head injured patients. Acta Neurochir. 1995;134(1–2):76–8.
Henker R, Brown S, Marion D, et al. Comparison of brain temperature with bladder and rectal temperatures in adults with severe head injury. Neurosurgery. 1998;42(5):1071–5.
Huschak G, Hoell T, Wiegel M, et al. Does brain temperature correlate with intracranial pressure? J Neurosurg Anesthesiol. 2008;20(2):105–9.
Picetti E, Oddo M, Prisco L, et al. A survey on fever monitoring and management in patients with acute brain injury: the SUMMA study. J Neurosurg Anesthesiol. 2019;31(4):399–405.
Steyerberg E, Wiegers E, Sewalt C, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18:923–34.
Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):1–106.
Carney N, Totten A, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15.
Zeiler F, Ercole A, Cabeleira M, et al. Comparison of performance of different optimal cerebral perfusion pressure parameters for outcome prediction in adult TBI: a CENTER-TBI Study. J Neurotrauma. 2019;36:1505–17.
Zeiler F, Ercole A, Cabeleira M, et al. Univariate comparison of performance of different cerebrovascular reactivity indices for outcome association in adult TBI: a CENTER-TBI study. Acta Neurochir. 2019;161:1217–27.
Steyerberg E, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165.
Qiu W, Shen H, Zhang Y, et al. Noninvasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. J Clin Neurosci. 2006;13:995–1000.
Oddo M, Frangos S, Milby A, et al. Induced normothermia attenuates cerebral metabolic distress in patients with aneurysmal subarachnoid hemorrhage and refractory Fever. Stroke. 2009;40(5):1913–6.
Rincon F, Hunter K, Schorr C, et al. The epidemiology of spontaneous fever and hypothermia on admission of brain injury patients to intensive care units: a multicenter cohort study. J Neurosurg. 2014;121(4):950–60.
Bao L, Chen D, Ding L, et al. Fever burden is an independent predictor for prognosis of traumatic brain injury. PLoS ONE. 2014;9:e90956.
Castillo J, Davalos A, Marrugat J, et al. Timing for fever-related brain damage in acute ischemic stroke. Stroke. 1998;29:2455–60.
Kazis A, Vlaikidis N, Xafenias D, et al. Fever and evoked potentials in multiple sclerosis. J Neurol. 1982;227:1–10.
Flensner G, Ek A, Söderhamn O, et al. Sensitivity to heat in MS patients: a factor strongly influencing symptomology—an explorative survey. BMC Neurol. 2011;11:27.
Cormio M, Citerio G, Spear S, et al. Control of fever by continuous, low-dose diclofenac sodium infusion in acute cerebral damage patients. Intensive Care Med. 2000;26:552–7.
Puccio A, Fischer M, Jankowitz B, et al. Induced normothermia attenuates intracranial hypertension and reduces fever burden after severe traumatic brain injury. Neurocrit Care. 2009;11:82–7.
Childs C, Vail A, Protheroe R, et al. Differences between brain and rectal temperatures during routine critical care of patients with severe traumatic brain injury. Anaesthesia. 2005;60:759–65.
Childs C, Wieloch T, Lecky F, et al. Report of a consensus meeting on human brain temperature after severe traumatic brain injury: its measurement and management during pyrexia. Front Neurol. 2010;23(1):96–103.
O’Grady N, Barie P, Bartlett J, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330–49.
Mcilvoy L. The impact of brain temperature and core temperature on intracranial pressure and cerebral perfusion pressure. J Neurosci Nurs. 2007;39(6):324–31.
Hawryluk G, Aguilera S, Buki A, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019; 1–12.
Ethical approval in CENTER-TBI; Ethical Approval. Accessed 2019. https://www.center-tbi.eu/project/ethical-approval.
Lahiri S, Sparrow N, Mangiacotti L, et al. 40th International Symposium on Intensive Care & Emergency Medicine. Crit Care. 2020;24(87). https://doi.org/10.1186/s13054-020-2772-3.
Acknowledgements
We are grateful to our patients with TBI for helping us in our efforts to improve care and outcome for TBI.
Collaboration group: CENTER-TBI High Resolution (HR ICU) Sub-Study Participants and Investigators: Audny Anke8, Ronny Beer9, Bo-Michael Bellander10, Erta Beqiri11, Andras Buki12,13, Manuel Cabeleira14, Arturo Chieregato11, Giuseppe Citerio15,16, Hans Clusmann17, Endre Czeiter18,19, Marek Czosnyka14, Bart Depreitere20, Ari Ercole21, Shirin Frisvold22, Stefan Jankowski23, Danile Kondziella24, Lars-Owe Koskinen25, Ana Kowark26, David K. Menon21, Geert Meyfroidt27, Kirsten Moeller28, David Nelson10, Anna Piippo-Karjalainen29, Andreea Radoi30, Arminas Ragauskas31, Rahul Raj29, Jonathan Rhodes32, Saulius Rocka31, Rolf Rossaint28, Juan Sahuquillo30, Oliver Sakowitz33,34, Nina Sundström35, Riikka Takala36, Tomas Tamosuitis37, Olli Tenovuo38, Peter Vajkoczy39, Alessia Vargiolu16, Rimantas Vilcinis40, Stefan Wolf41, Alexander Younsi34, Frederick A. Zeiler21,42
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. This research is funded by the European Commission 7th Framework program (602,150). Additional funding was obtained from the Hannelore Kohl Stiftung (Germany), from OneMind (USA), from Integra LifeSciences Corporation (USA), and from Neurotrauma Sciences (USA). The funders had no role in the design of the study and collection, analysis, interpretation of data and in writing the article. The datasets used and/or analyzed during the current study are available via https://www.center-tbi.eu/data on reasonable request.
Author information
Authors and Affiliations
Consortia
Contributions
FO, PS, MC, and TZ collected the patients’ data. TB, FO, and EW analyzed the data and drafted the tables and figures. TB and NS interpreted the data and drafted the article. TB, FO, and NS designed the study protocol, NS supervised the study. RH, SR, YS, MC, TZ, and BI were involved in regular meetings on the manuscript and reviewed the manuscript multiple times. All authors were involved in the design of the CENTER-TBI study and reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Birg reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), and grants from Neurotrauma Sciences (USA) during the conduct of the study. Dr. Ortolano reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), and grants from Neurotrauma Sciences (USA) during the conduct of the study. Dr. Wiegers reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), and grants from Neurotrauma Sciences (USA) during the conduct of the study. Dr. Smielewski reports grants from the European Commission 7th Framework program (602150) during the conduct of the study; personal fees from Cambridge Enterprise Ltd, Cambridge, UK, outside the submitted work. Dr. Savchenko reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), and grants from Neurotrauma Sciences (USA) during the conduct of the study. Dr. Ianosi reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study. Dr. Helbok reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study. Dr. Rossi reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study. Dr. Carbonara reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study. Dr. Zoerle reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study. Dr. Stochetti reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study.
Ethical approval/informed consent
In each recruiting site ethical approval was given; an overview is available online [43].
Clinical trial registration
ClinicalTrials.gov: NCT02210221.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is related to the Invited Commentary available at https://link.springer.com/article/10.1007/s12028-021-01295-0.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Birg, T., Ortolano, F., Wiegers, E.J.A. et al. Brain Temperature Influences Intracranial Pressure and Cerebral Perfusion Pressure After Traumatic Brain Injury: A CENTER-TBI Study. Neurocrit Care 35, 651–661 (2021). https://doi.org/10.1007/s12028-021-01294-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01294-1