Abstract
Background
Sodium chloride (NaCl) 23.4% solution has been shown to reduce intracranial pressure (ICP) and reverse transtentorial herniation. A limitation of 23.4% NaCl is its high osmolarity (8008 mOsm/l) and the concern for tissue injury or necrosis following extravasation when administered via peripheral venous access. The use of this agent is therefore often limited to central venous or intraosseous routes of administration. Our objective was to evaluate the safety and efficacy of administration of 23.4% NaCl via peripheral venous access compared with administration via central venous access.
Methods
We reviewed pharmacy records to identify all administrations of 23.4% NaCl at our institution between December 2017 and February 2020. Medical records were then reviewed to identify complications, such as extravasation, soft tissue injury or necrosis, hypotension (mean arterial pressure less than 65 mm Hg), pulmonary edema, hemolysis, and osmotic demyelination. We also compared the change in physiological variables, such as ICP, mean arterial pressure, cerebral perfusion pressure, and heart rate, as well as laboratory values, such as sodium, chloride, bicarbonate, creatinine, and hemoglobin, following administration of 23.4% NaCl via the peripheral and central venous routes.
Results
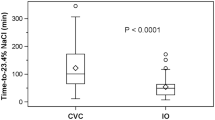
We identified 299 administrations of 23.4% NaCl (242 central and 57 peripheral) in 141 patients during the study period. There was no documented occurrence of soft tissue injury or necrosis in any patient. One patient developed hypotension following central administration. Among the 38 patients with ICP monitoring at the time of drug administration, there was no significant difference in median ICP reduction (− 13 mm Hg [central] vs. − 24 mm Hg [peripheral], p = 0.21) or cerebral perfusion pressure augmentation (16 mm Hg [central] vs. 15 mm Hg [peripheral], p = 0.87) based on route of administration.
Conclusions
Peripheral venous administration of 23.4% NaCl is safe and achieves a reduction in ICP equivalent to that achieved by administration via central venous access.
Similar content being viewed by others
References
Cook AM, Morgan Jones G, Hawryluk GWJ, Mailloux P, McLaughlin D, Papangelou A, et al. Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocrit Care. 2020;32(3):647–66.
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury. Fourth Ed Neurosurg. 2014;80(1):6–15.
Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(4):1222–38.
Koenig MA, Bryan M, Lewin JL 3rd, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70(13):1023–9.
Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of hypertonic (10%) saline in patients with raised intracranial pressure after stroke. Stroke. 2002;33(1):136–40.
Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery. 2005;57(4):727–36 (Discussion 727–36).
Suarez JI, Qureshi AI, Bhardwaj A, Williams MA, Schnitzer MS, Mirski M, et al. Treatment of refractory intracranial hypertension with 23.4% saline. Crit Care Med. 1998;26(6):1118–22.
Rockswold GL, Solid CA, Paredes-Andrade E, Rockswold SB, Jancik JT, Quickel RR. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurgery. 2009;65(6):1035–41 (Discussion 1041–32).
Alnemari AM, Krafcik BM, Mansour TR, Gaudin D. A comparison of pharmacologic therapeutic agents used for the reduction of intracranial pressure after traumatic brain injury. World Neurosurg. 2017;106:509–28.
Mangat HS, Chiu YL, Gerber LM, Alimi M, Ghajar J, Hartl R. Hypertonic saline reduces cumulative and daily intracranial pressure burdens after severe traumatic brain injury. J Neurosurg. 2015;122(1):202–10.
Dorman HR, Sondheimer JH, Cadnapaphornchai P. Mannitol-induced acute renal failure. Medicine (Baltimore). 1990;69(3):153–9.
Palma L, Bruni G, Fiaschi AI, Mariottini A. Passage of mannitol into the brain around gliomas: a potential cause of rebound phenomenon. A study on 21 patients. J Neurosurg Sci. 2006;50(3):63–6.
Al-Benna S, O’Boyle C, Holley J. Extravasation injuries in adults. ISRN Dermatol. 2013;2013:856541.
Wang J, Fang Y, Ramesh S, Zakaria A, Putman MT, Dinescu D, et al. Intraosseous administration of 23.4% NaCl for treatment of intracranial hypertension. Neurocrit Care. 2019;30(2):364–71.
Knight J, Rayi A. Transtentorial herniation. [Updated 2021 Feb 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560536/
Dornhofer P, Kellar JZ. Intraosseous vascular access. [Updated 2020 Jun 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554373/
Dillon RC, Merchan C, Altshuler D, Papadopoulos J. Incidence of adverse events during peripheral administration of sodium chloride 3. J Intensive Care Med. 2018;33(1):48–53.
Perez CA, Figueroa SA. Complication rates of 3% hypertonic saline infusion through peripheral intravenous access. J Neurosci Nurs. 2017;49(3):191–5.
Pancaro C, Shah N, Pasma W, Saager L, Cassidy R, van Klei W, et al. Risk of major complications after perioperative norepinephrine infusion through peripheral intravenous lines in a multicenter study. Anesth Analg. 2020;131(4):1060–5.
Jones GM, Bode L, Riha H, Erdman MJ. Safety of continuous peripheral infusion of 3% sodium chloride solution in neurocritical care patients. Am J Crit Care. 2016;26(1):37–42.
Baek SH, Jo YH, Ahn S, Medina-Liabres K, Oh YK, Lee JB, et al. Risk of overcorrection in rapid intermittent bolus vs slow continuous infusion therapies of hypertonic saline for patients with symptomatic hyponatremia: the SALSA randomized clinical trial. JAMA Intern Med. 2021;181(1):81–92.
Funding
No funding was provided for this work.
Author information
Authors and Affiliations
Contributions
LF made substantial contributions to design, acquisition of data, and analysis and interpretation of data and drafting the article. DH made substantial contributions to design, acquisition of data and critical revision for important intellectual content. SCR made substantial contributions to design and critical revision for important intellectual content. OK made substantial contributions to design and critical revision for important intellectual content. Craig A. Williamson Osama made substantial contributions to design and critical revision for important intellectual content. VR made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data and to drafting the article.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical Approval/Informed Consent
All ethical guidelines have been followed. Exemption from institutional review board oversight was confirmed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faiver, L., Hensler, D., Rush, S.C. et al. Safety and Efficacy of 23.4% Sodium Chloride Administered via Peripheral Venous Access for the Treatment of Cerebral Herniation and Intracranial Pressure Elevation. Neurocrit Care 35, 845–852 (2021). https://doi.org/10.1007/s12028-021-01248-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01248-7