Abstract
The appropriate use of medications during Emergency Neurological Life Support (ENLS) is essential to optimize patient care. Important considerations when choosing the appropriate agent include the patient’s organ function and medication allergies, potential adverse drug effects, drug interactions and critical illness and aging pathophysiologic changes. Critical medications used during ENLS include hyperosmolar therapy, anticonvulsants, antithrombotics, anticoagulant reversal and hemostatic agents, anti-shivering agents, neuromuscular blockers, antihypertensive agents, sedatives, vasopressors and inotropes, and antimicrobials. This article focuses on the important pharmacokinetic and pharmacodynamics characteristics, advantages and disadvantages and clinical pearls of these therapies, providing practitioners with essential drug information to optimize pharmacotherapy in acutely ill neurocritical care patients.
Similar content being viewed by others
Introduction
Neurocritical care patient management is very complicated, especially when trying to optimize therapy during acute injury. Pharmacologic management must be carefully considered in order to minimize cognitive dysfunction and avoid confounding patient evaluations. During emergency neurological life support (ENLS), pharmacotherapy must be individualized for each patient, taking into account their age, comorbidities, and chronic medications. Pharmacokinetic and pharmacodynamic characteristics must be considered as they may change in acute illness and with neurocritical care interventions, such as therapeutic hypothermia. Pharmacokinetic changes may include alterations in medication absorption, distribution, metabolism and elimination; while pharmacodynamics changes could result in loss of drug effect or toxicity. This chapter will focus on pharmacotherapy and clinical pearls that will help the ENLS provider optimize medication management in the acute period of neurological injury.
Hyperosmolar Therapy
Mannitol and hypertonic saline (HS) are commonly used in neurologically injured patients in the acute setting to treat elevated intracranial pressure (ICP) and cerebral edema. HS is also used in the treatment of hyponatremia. Both agents theoretically work by producing osmotically driven fluid shifts and appear to be equally effective at equal osmolar doses [1]. An online survey of neurointensivists reported that 90% use osmotic agents in the treatment of intracranial hypertension, with a fairly even split in preference for HS (55%) versus mannitol (45%) [2]. It is important to determine which agent would be best in individual patients based on serum sodium concentrations, plasma osmolality, fluid status, and renal function. A summary of the characteristics of these hyperosmolar agents can be found in Table 1.
Mannitol is an osmotic diuretic that is eliminated by the kidneys. Caution should be used in patients with kidney injury as mannitol may accumulate and worsen cerebral edema; especially in those with BBB disruption due to injury and/or inflammation. An osmolar gap is the most accurate monitoring tool to detect presence of unmeasured osmoles, such as mannitol, and should be used to monitor drug elimination between doses. An osmolar gap of 15–20 mOsm/kg indicates incomplete drug clearance between doses and increases risk of reverse osmotic shift and nephrotoxicity [3,4,5,6]. An osmolar gap can be calculated by subtracting the calculated osmolality from the measured osmolality. The laboratories (osmolality, BMP) necessary to calculate an osmolar gap should be drawn as a trough or prior to the mannitol dose. A plasma osmolality of >320 mOsm/kg is not a contraindication of ongoing administration of mannitol, as this is not a valid measure of excess mannitol and can also be increased with hyperglycemia. Urine output and electrolyte balances should also be carefully monitored to prevent hypotension, dehydration, and electrolyte imbalances due to excessive diuresis.
Unlike mannitol, HS provides fluid expansion and, therefore, patients with decompensated heart failure or pulmonary edema may be at increased risk of fluid overload. HS may have reduced risk of rebound cerebral edema after discontinuation due to the reflection coefficient of 1 vs 0.9 of mannitol [7, 8]. Caution should be used when administering to patients with chronic hyponatremia as the rapid change in serum sodium may increase the risk of osmotic demyelinating syndrome. Although there are some recommendations to use HS doses equiosmolar to mannitol, there are multiple other studies that show clinical benefit with variable concentrations, doses, and modes of administration of HS. Therefore, at this time, there is no information regarding optimal methods for use of HS. In general, established protocols allow for consistency of care among providers. For that reason, intensivists should work within their institution to come to a consensus regarding treatment goals, develop a protocol, monitor efficacy of the protocol, then re-assess and modify their protocol as needed to achieve the treatment goal. Trough serum sodium levels should be monitored prior to HS administration for HS dose and interval guidance, with the goal to use the lowest dose necessary [9].
Anticonvulsant Medications
Drug dosing can be complex when using anticonvulsant agents for status epilepticus (SE), seizure treatment or seizure prophylaxis in select disease states. Agents recommended for SE are given IV over a short period of time, but the choice of agent is mostly dependent on adverse drug reactions (ADRs) and patient stability. Many anticonvulsant agents contain propylene glycol in their intravenous formulation, which can cause hypotension during infusion and may accumulate with prolonged dosing causing metabolic acidosis and neurotoxicity. Anesthetic agents, such as pentobarbital, can cause ileus, immunosuppression, cardiotoxicity and hemodynamic instability and thus need to be monitored closely. Unfortunately, many institutions do not have therapeutic drug concentration monitoring available for pentobarbital and the kinetics are unpredictable; therefore, monitoring the agent’s effects with continuous EEG and hemodynamic devices is crucial. When treatment of neurological injury requires therapeutic hypothermia (TH), anticonvulsant agents that are metabolized by the liver will have prolonged duration of action. Careful anticonvulsant concentration monitoring is required and should be done approximately every 48 h over the duration of TH to avoid toxicity and for 3–5 days after rewarming as metabolism increases to baseline to avoid sub-therapeutic concentrations, with doses adjusted based on serum trough concentrations. Traumatic brain injury patients also tend to be hypermetabolic in the acute phase making dosing of anticonvulsant agents, as well as other hepatically metabolized medications, problematic. In these patients, drugs that are metabolized by the liver tend to have lower pharmacodynamics effects due to rapid metabolism and elimination, and higher doses may be required. Lastly, many of the first generation anticonvulsant agents have complex drug interactions that further require therapeutic drug monitoring and frequent assessment of ADRs. A summary of the medications used to treat SE can be found in Table 2.
Antithrombotic Agents
Antithrombotic agents may be used for management of acute ischemic stroke (AIS) in the first few hours (e.g. tissue plasminogen activator [tPA], aspirin [ASA], clopidogrel [Plavix]). In addition, patients presenting with intracranial hemorrhage may have be using ASA or Plavix for cardiovascular issues. Knowledge of the characteristics of these agents is important for patient safety. Due to unique mixing instructions (e.g. proper dilution, swirling vs shaking, etc.) of tPA, only those who have been educated on the process should reconstitute the drug. When preparing the appropriate dose of tPA, excess drug should be removed from the bottle before infusion to prevent inadvertent administration of total doses of >90 mg and increased risk of intracerebral bleeding. ASA can also be used acutely for patients who are not candidates for tPA; however, other antiplatelet agents, including prasugrel and ticagrelor, have a paucity of data for use acutely after ischemic stroke and prasugrel carries a warning for increased risk of serious bleeding in stroke patients. In patients who are allergic to aspirin and thus not candidates for tPA, clopidogrel can be used. Clopidogrel is commonly administered prior to endovascular procedures as a loading dose and then daily thereafter, and may be used in combination with ASA for 3 months in those who received intracranial stents. It should be noted that approximately 30% of the population have genetic polymorphisms and do not respond to clopidogrel as it is a prodrug that must be converted to its active form. Assays can be used to determine the effectiveness of platelet inhibition with ASA and clopidogrel.
Direct oral anticoagulant (DOACs) are used for stroke prevention in moderate to high risk patients with non-valvular atrial fibrillation. These agents inhibit either thrombin or factor Xa, which are essential for clot formation. DOACs have a significantly lower intracranial bleeding risk than warfarin, but are associated with a risk of gastrointestinal bleeding. When considering tPA for a patient with an AIS, DOAC administration within the last 48 h or any abnormal coagulation tests for these specific agents are a contraindication for receiving tPA. The time of last dose and renal function is essential to know if your institution does not have the specific assays to evaluate each drug class. If the patient requires ASA for AIS and is on a DOAC, there is an increased risk of bleeding and the risk to benefit ratio must be considered until the DOAC has had time to be cleared from the body (i.e. approximately 3–5 half-lives).
The pharmacologic properties of the warfarin, DOACs and antiplatelet agents commonly used in AIS can be found in Tables 3 and 4.
Anticoagulant/Antiplatelet Reversal and Hemostatic Agents
Reversal of anticoagulants in patients with life threatening bleeding or sustained bleeding is critical. Life threatening bleeding can include intracerebral hemorrhage, gastrointestinal bleeding, uncontrolled retroperitoneal or any hemorrhage into an extremity with risk of compartment syndrome. Reversal may also be necessary when an emergent surgical intervention is required within 6–12 h of presentation. General management strategies to employ when treating major hemorrhages include identifying the cause and source of bleeding, maintaining hemodynamic and respiratory stability, maintaining normal body temperature, blood pH and electrolyte balance to facilitate coagulation, application of packing or dressing if applicable, local hemostatic measures or surgical intervention to control bleeding, and lastly identify the anticoagulant and administer an appropriate reversal agent [20].
When reversing an anticoagulant, the risk of continued bleeding to risk of thrombosis is of the utmost importance and should be determined in each case. Second, timing of the last dose of anticoagulant administered and elimination half-life are also necessary to determine if reversal is warranted. If the agent was taken within the 3–5 half-life window, then reversal should be considered in patients with a higher risk of continued bleeding [21]. In medications with longer half-lives (i.e. apixaban) reversal may be considered out to 2–3 days from the last dose. When considering all oral anticoagulants, if the oral agent has been ingested in the previous 2 h, 50 g of oral activated charcoal should be considered. Risks and benefits of this reversal strategy should be considered, especially in patients with gastrointestinal bleeding.
When reversing warfarin the administration of both a rapid reversal agent as well as an agent with sustained effect is crucial since the half-live of warfarin is fundamentally the half-life of factors II (42–72 h), VII (4–6 h), IX (21–30 h), and X (27–48 h). For rapid reversal of warfarin, the Neurocritical Care Society (NCS), American Colleges of Chest Physicians, (CHEST) and American Heart Association/American Stroke Association (AHA/ASA) guidelines suggest use of prothrombin complex concentrate (PCC) agents over fresh frozen plasma (FFP) [22,23,24]. In addition, a recent study showed 4-Factor PCC to be more likely to achieve a reduction of INR to <1.3 in 3 h and was associated with less hematoma expansion than FFP in warfarin-associated ICH patients [25]. PCC’s are generally better tolerated than FFP due to lower fluid volumes and decreased risk for transfusion related acute lung injury (TRALI) or circulatory overload (TACO) [26, 27]. There are two types of PCC products available, 4-factor and 3-factor PCC. 4-factor PCC contain all of the vitamin K-dependent coagulation factors, are sufficient to provide immediate warfarin reversal and are the preferred products when available. 3-factor PCC contains only factors II, IX, and X and consideration should be made to supplement with FFP or recombinant factor VIIa to completely reverse anticoagulation. FFP may be a better choice however in patients that require volume resuscitation, and may be used in combination with PCC if reversal is inadequate. When administering PCC for reversal of warfarin, the dose administered is generally based on the clinical scenario and INR (see Table 5). A repeat INR may be checked 30 min after the end of PCC infusion to evaluate if it is within normal range [26, 28,29,30]. Although limited data is available for recommending a second dose of PCC, if INR remains elevated and risk of continued bleeding is still high, a second dose of PCC may be considered. Of note, factor eight inhibitor bypassing agent (FEIBA®), a 4-factor PCC with activated factor VII, may interfere with INR test resulting in a falsely low INR. Four-factor PCC was recently shown to be more effective than FFP in reversing INR, especially when reversal is emergent [31]. There are no clinical trials combining PCC and factor rVIIa for treatment of life threatening bleeding. It is unknown if combined use increases efficacy or risk of thrombosis. Current recommendation is to not combine factor rVIIa and 4-factor PCC. For sustained reversal, phytonadione (vitamin K) should be simultaneously administered to patients who require rapid reversal. Intravenous and oral vitamin K effectively lowers INR within 12–14 and 24–36 h, respectively [32,33,34]. Subcutaneous administration is not recommended due to unpredictable absorption and delayed response [35].
Idarucizumab (Praxbind) is a humanized monoclonal antibody fragment that binds dabigatran to neutralize its anticoagulant effects within minutes of administration. The recommended dose of idarucizumab is 5 g intravenously, administered in two 2.5 g doses [36,37,38]. Each 2.5 g vial should be administered over no longer than 5–10 min and each vial should be given no more than 15 min apart. Idarucizumab must be administered within 1 h after removal from the vial. It has been demonstrated that plasma dabigatran concentrations may rebound 12–24 h after idarucizumab administration, likely due to re-distribution from the extravascular compartment. Safety and effectiveness of repeat treatment with idarucizumab has not been established. Additionally, renal impairment does not impact the reversal effect of idarucizumab and therefore there is no dose adjustment necessary in patients with renal impairment.
Until the release of a specific antidote to reverse factor-Xa inhibitors, administration of a 4-factor PCC should be strongly considered for reversal of life-threatening bleeds in patients taking factor-Xa inhibitors (apixaban, rivaroxaban, edoxaban). There is no standard recommended dose of PCC for treatment of bleeding associated with these agents, but one study demonstrated an improvement in surrogate endpoints, endogenous thrombin potential and PTT, in health individuals who received PCC 50 units/kg to reverse rivaroxaban [39]. Only animal studies have evaluated factor VIIa for treatment of bleeding associated with rivaroxaban. Andexanet alfa is a recombinant, inactive protein analogue of factor-Xa that competitively binds apixaban and rivaroxaban and eliminates the ability of these agents to inhibit endogenous factor-Xa. Andexanet alfa should be available in the United Stated by the end of 2017 and will be the drug of choice for reversal of life threatening bleeding in patients taking factor-Xa inhibitors. Studies have shown the intravenous administration of a bolus of andexanet, followed by 2 h of continuous infusion, restores endogenous factor-Xa function with effective hemostasis in patients with acute major bleeding receiving oral factor-Xa inhibitors [40, 41].
Recombinant activated factor VII (rFVIIa) was shown to decrease hematoma growth in non-coagulopathic ICH patients, however no improvement in mortality was demonstrated in a large randomized trial so it is not recommended in this patient population [42]. The NCS, CHEST and AHA/ASA do not recommend rFVIIa for warfarin reversal although its use to supplement 3-factor PCC in patients with life threatening bleeding on warfarin may be considered [24, 43,44,45]. The dose of rFVIIa is not well established and generally lower doses (10–20 mcg/kg) are preferred due to risk of thrombosis with higher doses. The duration of INR correction is dose dependent, transient, and does not reflect efficacy. Furthermore, there may be an increased risk of thrombotic complications with the use of rFVIIa and this risk may be increased with concomitant use of PCC although not well established.
Platelet transfusions are used commonly for both prophylactic and therapeutic reversal of antiplatelet therapy in patients taking an antiplatelet agent (Aspirin, clopidogrel, prasugrel, ticagrelor) with acute neurologic injury. Although a paucity in the literature still exists in regards to administration of platelets for an emergent neurosurgical procedure or in patients after TBI or aSAH, recent findings from the PATCH trail demonstrate worse outcomes in patients that receive platelets for spontaneous ICH when compared to standard care without platelet therapy [46]. However, few patients in PATCH were on clopidogrel and those undergoing neurosurgical procedures were excluded. (Please see ENLS ICH module for further recommendations regarding platelet transfusion) (Tables 6, 7, 8, 9).
Antifibrinolytic Therapy after Subarachnoid Hemorrhage
Re-hemorrhage is a significant problem in management of patients with aneurysmal subarachnoid hemorrhage (SAH) and contributes to morbidity and mortality in the acute setting. Antifibrinolytics have a role as short term therapy (less than 72 h) to prevent rebleeding in the acute setting while waiting for treatment to secure the aneurysm. Several retrospective and one prospective study have shown that a short course of an antifibrinolytic reduce the rate of re-hemorrhage without an increase in cerebral ischemia, vasospasm, and/or hydrocephalus [49,50,51].
Tranexamic acid is generally dosed as 1 g IV given over 10 min every 4–6 h and aminocaproic acid (Amicar) as a 5 g IV bolus given over 1 h followed by 1 g/h infusion. Caution must be used when giving concomitantly with nimodipine as both may cause a precipitous decrease in blood pressure. Due to potential risk of ischemic complications in patients undergoing endovascular treatment, one may consider holding antifibinolytic therapy 4–6 h prior to the endovascular procedure to prevent thrombotic complications. Only short-term therapy, generally less than 72 h post bleed, is recommended.
Shiver Control during Therapeutic Temperature Management
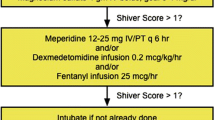
Shivering is a physiologic homeostatic mechanism that helps maintain temperature and is triggered in humans when core temperature falls below 36 degrees Celsius and ceases at temperatures <34 Celsius. Elderly patients have approximately a 1-degree Celsius lower shivering threshold than younger patients [52]. Sustained shivering increases the metabolic rate and should be avoided as it counteracts cooling induction, consumes energy, contributes to increased intracranial pressure, and increases brain oxygen consumption [53]. Therefore it is crucial to evaluate and treat shivering in patients who are being treated with therapeutic temperature management (Tables 10, 11; Fig. 1).
Neuromuscular Blocking Agents
Neuromuscular blocking agents are used as an adjunct to general anesthesia to facilitate tracheal intubation, provide skeletal muscle relaxation during surgery, facilitate mechanical ventilation, assist in treatment of malignant ICP, or control refractory shivering during targeted temperature management. Short acting agents are preferred. These agents interrupt signal transmission at the neuromuscular junction, and are categorized as either depolarizing or non-depolarizing agents. Succinylcholine is the only depolarizing paralytic and works by mimicking the action of acetylcholine. All other agents are non-depolarizing, competitive acetylcholine antagonists. Drug Interactions can be seen with drugs that reduce or inhibit plasma cholinesterase activity, and synergistic effects may occur with other neuromuscular blocking agents. Certain medications can increase the duration of neuromuscular blockade. (Paralytics affect all skeletal muscles but have no effect on consciousness, and therefore must be used with proper sedation and analgesia. Monitoring the train-of-four (TOF) with a peripheral nerve stimulator (PNS) in conjunction with the clinical assessment (vital signs, synchrony with the mechanical ventilator) should always be utilized to evaluate the extent of paralysis. The TOF goal is generally 1–2 responses per 4 stimulations. Caution should be used when using a PNS in hypothermic patients as the TOF may be unreliable and misleading. Therefore, caution should be exercised when using PNS in the setting of hypothermia (Tables 12, 13).
Sedation and Analgesia
When using sedative and analgesic agents during ENLS, treatment and monitoring goals must be identified and communicated. Many of these agents will be affected by end organ dysfunction and drug interactions, so choices must be individualized for each patient based on these parameters. The minimum effective dose should be used. When used in combination, many of these agents are synergistic, so lower doses of both agents can be used (e.g. propofol and morphine). Older adult patients may be more sensitive to these agents and have impaired renal and hepatic function that prolongs drug effects, thus lower doses and shorter acting agents are preferred. Sedatives and opiates may produce tolerance and dependence, which can result in increasing dose requirements. Withdrawal symptoms can occur with abrupt discontinuation after prolonged use; therefore, it is suggested to down titrate maintenance infusions by not more than 25% daily.
Drug interactions for these agents can lead to further complications, overutilization of monitoring devices and longer intensive care unit (ICU) lengths of stay. Monitoring medications as they are added and discontinued can help prevent unwanted adverse drug reactions. Sedative and analgesic agents commonly used in the ICU can be found in Tables 14 and 15.
Intravenous Antihypertensive Agents
Intravenous antihypertensive are necessary to mitigate hypertension in many acute neurologic conditions. Blood pressure goals vary dramatically between disease states and controversy surrounds the definition of best practice in many areas. When blood pressure reduction is required the agent of choice should be selected based on the rapidity of control required, underlying cardiovascular function, volume status, organ function, and other hemodynamic parameters (i.e. heart rate) and drug interactions (Table 16).
Vasopressors and Inotropes
Vasopressor agents induce vasoconstriction and elevation of mean arterial pressure. They are used in the neurological patient in a variety of situations when blood pressure augmentation is desired to treat shock, vasospasm or improve cerebral or spinal perfusion pressure. Vasopressors produce their effects through their actions at adrenergic (alpha and beta), dopamine and vasopressin receptors in the body (Table 17). Alpha-1 adrenergic receptors are located in vascular walls and the heart. Activation of these receptors leads to significant vasoconstriction and increased duration of cardiac contraction. Beta-1 adrenergic receptors are most common in the heart and activation has inotropic and chronotropic effects with minimal vasoconstriction. Beta-2 adrenergic receptors are located in blood vessels, and activation induces vasodilation. Dopamine receptors are present in cerebral, coronary, renal, and mesenteric vascular beds. Activation of these receptors generally leads to vasodilation, although there is a second subtype of dopamine receptors that can cause vasoconstriction through release of norepinephrine as the dose of dopamine increases.
Vasopressin (antidiuretic hormone) is a non-adrenergic vasopressor that is used in diabetes insipidus and as a second-line agent in refractory shock. It may also allow a reduction in the required dose of first-line vasopressors. Adverse effects include hyponatremia, which may worsen cerebral edema, and pulmonary vasoconstriction contributing to hypoxia. Milrinone is another non-adrenergic agent that has both inotropic and vasodilatory effects. It is a phosphodiesterase inhibitor that can be used to provide cardiac support, but its vasodilatory effects may worsen hypotension.
Few comparative studies of these agents have been performed [62, 63], so one vasopressor cannot be recommended over others; thus selection of which agent to use must be based on goals of care and desired physiologic effects.
Antibiotics
When treating meningitis and encephalitis, choosing an appropriate antimicrobial or antiviral agent and the appropriate dose is essential. Most antibiotics are hydrophilic and do not cross the blood–brain-barrier (BBB) well. However, when the meninges are inflamed, penetration increases and allows drug to reach the site of action. Antibiotics and their microbial targets are listed in Table 18. Steroids (e.g. dexamethasone) are sometimes used in conjunction with antibiotics for S. pneumonia meningitis to decrease neurological sequelae [64]. If used, dexamethasone 10 mg intravenously every 6 h × 4 days is recommended and should be given before or at the same time as the first antibiotic dose.
Conclusion
Pharmacologic management in patients undergoing ENLS is very challenging, especially while attempting to minimize further cognitive dysfunction and worse outcomes. Medication choices and doses must be individualized for each patient, taking into account their medical history, comorbidities, pharmacokinetic and pharmacodynamic changes due to age, critical illness, and neurocritical care interventions; potential adverse drug effects, and drug interactions. Appropriate pharmacotherapy is essential in optimizing care in the patients with neurological emergencies.
References
Kamel H, Cornes SB, Hegde M, Hall SE, Josephson SA. Electroconvulsive therapy for refractory status epilepticus: a case series. Neurocrit Care. 2010;12(2):204–10.
Hays AN, Lazaridis C, Neyens R, Nicholas J, Gay S, Chalela JA. Osmotherapy: use among neurointensivists. Neurocrit Care. 2011;14(2):222–8.
Perez-Perez AJ, Pazos B, Sobrado J, Gonzalez L, Gandara A. Acute renal failure following massive mannitol infusion. Am J Nephrol. 2002;22(5–6):573–5.
Gadallah MF, Lynn M, Work J. Case report: mannitol nephrotoxicity syndrome: role of hemodialysis and postulate of mechanisms. Am J Med Sci. 1995;309(4):219–22.
Dorman HR, Sondheimer JH, Cadnapaphornchai P. Mannitol-induced acute renal failure. Medicine. 1990;69(3):153–9.
Gondim Fde A, Aiyagari V, Shackleford A, Diringer MN. Osmolality not predictive of mannitol-induced acute renal insufficiency. J Neurosurg. 2005;103(3):444–7.
Rudehill A, Gordon E, Ohman G, Lindqvist C, Andersson P. Pharmacokinetics and effects of mannitol on hemodynamics, blood and cerebrospinal fluid electrolytes, and osmolality during intracranial surgery. J Neurosurg Anesthesiol. 1993;5(1):4–12.
Palma L, Bruni G, Fiaschi AI, Mariottini A. Passage of mannitol into the brain around gliomas: a potential cause of rebound phenomenon. A study on 21 patients. J Neurosurg Sci. 2006;50(3):63–6.
Kheirbek T, Pascual JL. Hypertonic saline for the treatment of intracranial hypertension. Curr Neurol Neurosci Rep. 2014;14(9):482.
Forsyth LL, Liu-DeRyke X, Parker D Jr, Rhoney DH. Role of hypertonic saline for the management of intracranial hypertension after stroke and traumatic brain injury. Pharmacotherapy. 2008;28(4):469–84.
Papangelou A, Lewin JJ 3rd, Mirski MA, Stevens RD. Pharmacologic management of brain edema. Curr Treat Options Neurol. 2009;11(1):64–73.
Roquilly A, Mahe PJ, Latte DD, Loutrel O, Champin P, Di Falco C, et al. Continuous controlled-infusion of hypertonic saline solution in traumatic brain-injured patients: a 9-year retrospective study. Crit Care. 2011;15(5):R260.
Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23.
Lexi-Comp IL-D. Lexi-Comp, Inc; 2015.
Ubogu EE, Sagar SM, Lerner AJ, Maddux BN, Suarez JI, Werz MA. Ketamine for refractory status epilepticus: a case of possible ketamine-induced neurotoxicity. Epilepsy Behav: E&B. 2003;4(1):70–5.
Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(1):227–76.
Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45(2):246–51.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44–122.
Lexi-Comp IL-D. Lexi-Comp, Inc; 2013.
Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65(4):951–60.
Crowther MA, Warkentin TE. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost: JTH. 2009;7(Suppl 1):107–10.
Leissinger CA, Blatt PM, Hoots WK, Ewenstein B. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol. 2008;83(2):137–43.
Tran H, Collecutt M, Whitehead S, Salem HH. Prothrombin complex concentrates used alone in urgent reversal of warfarin anticoagulation. Int Med J. 2011;41(4):337–43.
Frontera JA, Lewin JJ 3rd, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: a statement for healthcare professionals from the neurocritical care society and society of critical care medicine. Neurocrit Care. 2016;24(1):6–46.
Steiner T, Poli S, Griebe M, Husing J, Hajda J, Freiberger A, et al. Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol. 2016;15(6):566–73.
van Aart L, Eijkhout HW, Kamphuis JS, Dam M, Schattenkerk ME, Schouten TJ, et al. Individualized dosing regimen for prothrombin complex concentrate more effective than standard treatment in the reversal of oral anticoagulant therapy: an open, prospective randomized controlled trial. Thromb Res. 2006;118(3):313–20.
Crawford JH, Augustson BM. Prothrombinex use for the reversal of warfarin: is fresh frozen plasma needed? Med J Aust. 2006;184(7):365–6.
Dager WE. Using prothrombin complex concentrates to rapidly reverse oral anticoagulant effects. Ann Pharmacother. 2011;45(7–8):1016–20.
Bershad EM, Suarez JI. Prothrombin complex concentrates for oral anticoagulant therapy-related intracranial hemorrhage: a review of the literature. Neurocrit Care. 2010;12(3):403–13.
Holland L, Warkentin TE, Refaai M, Crowther MA, Johnston MA, Sarode R. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion. 2009;49(6):1171–7.
Goldstein JN, Refaai MA, Milling TJ Jr, Lewis B, Goldberg-Alberts R, Hug BA, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385:2077–87.
Weibert RT, Le DT, Kayser SR, Rapaport SI. Correction of excessive anticoagulation with low-dose oral vitamin K1. Ann Intern Med. 1997;126(12):959–62.
Brophy MT, Fiore LD, Deykin D. Low-dose vitamin K therapy in excessively anticoagulated patients: a dose-finding study. J Thromb Thrombolysis. 1997;4(2):289–92.
Lubetsky A, Yonath H, Olchovsky D, Loebstein R, Halkin H, Ezra D. Comparison of oral vs intravenous phytonadione (vitamin K1) in patients with excessive anticoagulation: a prospective randomized controlled study. Arch Intern Med. 2003;163(20):2469–73.
Nee R, Doppenschmidt D, Donovan DJ, Andrews TC. Intravenous versus subcutaneous vitamin K1 in reversing excessive oral anticoagulation. Am J Cardiol. 1999;83(2):286–8 (A6–7).
Pollack CV Jr. Evidence supporting idarucizumab for the reversal of dabigatran. Am J Emerg Med. 2016;34(11S):33–8.
Reilly PA, van Ryn J, Grottke O, Glund S, Stangier J. Idarucizumab, a specific reversal agent for dabigatran: mode of action, pharmacokinetics and pharmacodynamics, and safety and efficacy in phase 1 subjects. Am J Emerg Med. 2016;34(11S):26–32.
Teleb M, Salire K, Wardi M, Alkhateeb H, Said S, Mukherjee D. Idarucizumab: clinical role of a novel reversal agent for dabigatran. Cardiovasc Hematol Disord Drug Targets. 2016;16(1):25–9.
Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–9.
Wang JJ, Gosselin S, Villeneuve E. Andexanet alfa for factor Xa inhibitor reversal. N Engl J Med. 2016;375(25):2498.
Connolly SJ, Milling TJ Jr, Eikelboom JW, Gibson CM, Curnutte JT, Gold A, et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131–41.
Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127–37.
Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, Connolly ES Jr, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–29.
Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e152S–84S.
Baglin TP, Keeling DM, Watson HG. Guidelines on oral anticoagulation (warfarin): third edition—2005 update. Br J Haematol. 2006;132(3):277–85.
Baharoglu MI, Cordonnier C, Al-Shahi Salman R, de Gans K, Koopman MM, Brand A, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387(10038):2605–13.
Monte AA, Bodmer M, Schaeffer TH. Low-molecular-weight heparin overdose: management by observation. Ann Pharmacother. 2010;44(11):1836–9.
Bordes J, Asencio Y, Kenane N, Fesselet J, Meaudre E, Goutorbe P. Recombinant activated factor VII for acute subdural haematoma in an elderly patient taking fondaparinux. Br J Anaesth. 2008;101(4):575–6.
Harrigan MR, Rajneesh KF, Ardelt AA, Fisher WS 3rd. Short-term antifibrinolytic therapy before early aneurysm treatment in subarachnoid hemorrhage: effects on rehemorrhage, cerebral ischemia, and hydrocephalus. Neurosurgery. 2010;67(4):935–9 ; discussion 9–40.
Schuette AJ, Hui FK, Obuchowski NA, Walkup RR, Cawley CM, Barrow DL, et al. An examination of aneurysm rerupture rates with epsilon aminocaproic acid. Neurocrit Care. 2013;19(1):48–55.
Starke RM, Kim GH, Fernandez A, Komotar RJ, Hickman ZL, Otten ML, et al. Impact of a protocol for acute antifibrinolytic therapy on aneurysm rebleeding after subarachnoid hemorrhage. Stroke. 2008;39(9):2617–21.
Kurz A, Plattner O, Sessler DI, Huemer G, Redl G, Lackner F. The threshold for thermoregulatory vasoconstriction during nitrous oxide/isoflurane anesthesia is lower in elderly than in young patients. Anesthesiology. 1993;79(3):465–9.
Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008;39(12):3242–7.
Mokhtarani M, Mahgoub AN, Morioka N, Doufas AG, Dae M, Shaughnessy TE, et al. Buspirone and meperidine synergistically reduce the shivering threshold. Anesth Analg. 2001;93(5):1233–9.
Talke P, Tayefeh F, Sessler DI, Jeffrey R, Noursalehi M, Richardson C. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology. 1997;87(4):835–41.
Lysakowski C, Dumont L, Czarnetzki C, Tramer MR. Magnesium as an adjuvant to postoperative analgesia: a systematic review of randomized trials. Anesth Analg. 2007;104(6):1532–9 (table of contents).
Badjatia N, Kowalski RG, Schmidt JM, Voorhees ME, Claassen J, Ostapkovich ND, et al. Predictors and clinical implications of shivering during therapeutic normothermia. Neurocrit Care. 2007;6(3):186–91.
Murray MJ, DeBlock H, Erstad B, Gray A, Jacobi J, Jordan C, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically Ill patient. Crit Care Med. 2016;44(11):2079–103.
Flower O, Hellings S. Sedation in traumatic brain injury. Emerg Med Int. 2012;2012:637171.
Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–41.
Devlin JW, Roberts RJ. Pharmacology of commonly used analgesics and sedatives in the ICU: benzodiazepines, propofol, and opioids. Crit Care Clin. 2009;25(3):431–49 (vii).
Mullner M, Urbanek B, Havel C, Losert H, Waechter F, Gamper G. Vasopressors for shock. Cochrane Database Syst Rev. 2004;3:CD003709.
Sookplung P, Siriussawakul A, Malakouti A, Sharma D, Wang J, Souter MJ, et al. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit Care. 2011;15(1):46–54.
van de Beek D, de Gans J, McIntyre P, Prasad K. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2007;1:CD004405.
van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362(2):146–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Gretchen Brophy is an advisory board member/consultant/speaker for UCB, Edge Therapeutics, Chiesi, Sage and Mallinckrodt; has received research funding from Mallinckrodt; and is an executive officer for the Neurocritical Care Society. Dr. Theresa Human is an advisory board member/consultant/speaker for Cumberland Pharmacueticals, UCB, and is a Member of the Board of Directors and Co-Chair of the Educational Products Committee for the Neurocritical Care Society.
Rights and permissions
About this article
Cite this article
Brophy, G.M., Human, T. Pharmacotherapy Pearls for Emergency Neurological Life Support. Neurocrit Care 27 (Suppl 1), 51–73 (2017). https://doi.org/10.1007/s12028-017-0456-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-017-0456-x