Abstract
Background
Early unplanned readmissions of “bouncebacks” to intensive care units are a healthcare quality metric and result in higher mortality and greater cost. Few studies have examined bouncebacks to the neurointensive care unit (neuro-ICU), and we sought to design and implement a quality improvement pilot to reduce that rate.
Methods
First, we performed a retrospective chart review of 504 transfers to identify potential bounceback risk factors. Risk factors were assessed on the day of transfer by the transferring physician identifying patients as “high risk” or “low risk” for bounceback. “High-risk” patients underwent an enhanced transfer process emphasizing interdisciplinary communication and rapid assessment upon transfer during a 9-month pilot.
Results
Within the retrospective cohort, 34 of 504 (4.7%) transfers required higher levels of care within 48 h. Respiratory failure and sepsis/hypotension were the most common reasons for bounceback among this group. During the intervention, 8 of 225 (3.6%) transfers bounced back, all of who were labeled “high risk.” Being “high risk” was associated with a risk of bounceback (OR not calculable, p = 0.02). Aspiration risk (OR 6.9; 95% CI 1.6–30, p = 0.010) and cardiac arrhythmia (OR 7.1; 95% CI 1.6–32, p = 0.01) were independent predictors of bounceback in multivariate analysis. Bounceback rates trended downward to 2.8% in the final phase (p for trend 0.09). Eighty-five percent of providers responded that the pilot should become standard of care.
Conclusion
Patients at high risk for bounceback after transfer from the neuro-ICU can be identified using a simple tool. Early augmented multidisciplinary communication and care for high-risk patients may improve their management in the hospital.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intensive care unit (ICU) readmission rates are used as healthcare quality measurements by several critical care organizations including the Society of Critical Care Medicine and the European Society of Intensive Care Medicine [1, 2]. Patients who require readmission to an ICU within 48 h of transfer have been shown to have longer length of stays, increased mortality, and higher overall cost to the healthcare system [3,4,5,6,7,8]. Factors that result in unplanned return to the ICU or “bounceback” likely reflect a combination of issues that are patient-specific, disease-specific, and healthcare system-specific. Several small studies have evaluated patients from medical and surgical ICUs and reasons for their bouncebacks. Only one study to date has examined unplanned readmissions to a neurointensive care unit (neuro-ICU) [9].
Across these studies, the most commonly identified factors leading to ICU readmissions in these populations are respiratory distress/hypoxia, cardiac arrhythmias, and sepsis [3,4,5, 9,10,11,12,13], and several composite scores of acuity and illness have been examined [14,15,16,17]. Other parameters that have been investigated but yield varying results are time of transfer [10, 18, 19] and measures of ICU stress and acuity [11, 20]. The reported incidence of bouncebacks within 48 h in these studies varies between 3 and 11% [9, 12, 21,22,23]. A variety of interventions have been implemented to address ICU bounceback rates including standardized transfer checklists [12], ICU outreach programs and clinical liaisons [24,25,26,27], and integration of several services around the time of transfer [28, 29]. The design of these projects may be partially dependent on local factors [30]. To date, there has been little published regarding patients in a neuro-ICU [9], who may have unique risk factors predisposing them to bounceback. We hypothesized that we could identify key neuromedical factors that would identify which neurology patients are at greatest risk of bounceback after transfer and then use this information to design and implement a pilot program using quality improvement techniques that would emphasize enhanced communication and early evaluation of those high-risk patients.
Methods
Setting and Population
The study took place in a large urban academic tertiary care hospital with a 22 bed neuro-ICU that cares for both neurosurgical and neurology patients. After care in the neuro-ICU, patients were transitioned to one of three specialty units within the hospital, two of which were floor units with a 1:4–5 nursing ratio and one of which was a step-down unit with a 1:2 nursing ratio and capability to manage stable ventilator settings and certain IV medications. This study analyzed only the neurology patient population. All patients transferred from the neuro-ICU to the neurology inpatient services were included in this quality initiative. We excluded patients who were discharged home from the ICU, died in the ICU, were transferred on hospice care or with comfort care only measures, or were transferred from the neuro-ICU to a service other than neurology.
We performed a retrospective analysis of the cohort of neurology patients transferred out of the neuro-ICU during the period July 1, 2012 to October 27, 2014. For the purposes of this study, a bounceback was defined as either an unplanned return to the neuro-ICU or an unplanned transfer from a regular floor bed to the step-down unit within 48 h of transfer out of the neuro-ICU, since both of these represent a need for an increased level of care after transfer. Forty-eight hours were chosen based on the results of previous studies that looked at the optimum time to analyze ICU readmissions [14]. Among those who bounced back, each medical record was reviewed by a clinician (DC) to identify the primary reason for the bounceback. We also collected basic demographic data, clinical data including primary diagnosis, ICU length of stay, hospital length of stay, time of transfer, time of bounceback, and post-hospitalization disposition. Post-hospitalization disposition was grouped into the following categories: home, acute rehabilitation facility, skilled nursing facility/other, or death/hospice care. ICU length of stay, hospital length of stay, time of transfer, age, and sex were compared between the two groups using independent sample t-test. Post-hospitalization disposition for bouncebacks versus non-bouncebacks was compared using Pearson’s chi-squared test using categories of home, acute care rehabilitation facility, skilled nursing facility, hospice, and others. Patients were excluded from analysis if they were transferred to a non-neurology service, if they died in the neuro-ICU, or if they stayed in the neuro-ICU for less than 24 h.
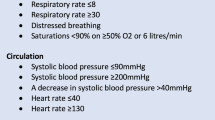
Based on the retrospective analysis, we created a checklist to identify patients who may be at high risk for bouncing back within 48 h of transfer (see Supplemental Data). Potential risk factors based on the retrospective analysis and face validity included duration of mechanical ventilation for ≥4 days, extubation within the prior 24 h, failed extubation requiring reintubation during the ICU course, high aspiration risk, any seizure within the last 24 h, depressed or fluctuating mental status, active diuresis within 48 h, initiation or broadening of antibiotic therapy within 24 h, any cardiac arrhythmia, hypotension requiring treatment within last 24 h, hypertension requiring antihypertensive infusion or frequent bolus administration, neurosurgical or interventional radiology procedure within last 24 h, and any history of prior neuro-ICU bounceback.
Extensive stakeholder analysis was performed from January 2015 to April 2015 with interdisciplinary discussions among physicians, nurses, and respiratory therapists representing both the neuro-ICU and the floor teams to inform the structure of the intervention. The checklist was completed on the day of transfer and was used to characterize patients as either “high risk” of decompensation within 48 h of transfer (≥1 factor present) or “low risk” (0 factors present). Those who were “high risk” underwent a specialized transfer process involving neuro-ICU physicians, nurses, and respiratory therapists involving communication between physicians, nurses, respiratory therapists and their floor counterparts, where enhanced communication emphasized risk status and specific risk factors. High-risk patients were identified and appropriately designated in the hospital’s bed management software system (NavicareTM) by a highly visualized green bar, and upon transfer to the floor, automatic text alerts were sent to the covering senior resident and on-call respiratory therapists. “High-risk” patients were evaluated within 1 h by the receiving physician, and within 2 h by a respiratory therapist (see Fig. 1). The checklist itself, readily identifiable by its bright green-colored paper, would remain prominently posted in a patient’s room afterward for at least 72 h as a visual reminder. This process was adapted from a recent quality improvement initiative in our surgical ICU [31]. Education for nursing, physicians, and respiratory therapists occurred during May and June of 2015. This intervention was implemented in three, 3-month stages between July 1, 2015 and April 1, 2016. In stage 1, only physicians used the checklist and integrated risk status into their transfer process. In stage 2, nurses began filling out their sections of the checklist and integrating risk status into their handoffs in addition to physicians. In stage 3, respiratory therapists were added and automatic text message system was implemented.
Outcomes for the intervention included the rate of bouncebacks, ICU length of stay, rates of compliance with the pilot, and time to assessment by floor providers and floor respiratory therapists. Rates of bounceback during the intervention were compared to the bounceback rate in the retrospective portion using independent sample t-test. Hospital length of stay and ICU length of stay were also compared to the pre-intervention values. Performance of the tool was assessed by establishing receiver operating curves at a variety of risk factor cutoffs. Individual risk factors as well as the checklist’s overall ability to predict bouncebacks were assessed. A questionnaire was delivered to physicians, nurses, and respiratory therapists involved with the pilot to assess subjective impact on care delivery and assess further areas for process improvement.
Results
Part I: Retrospective Analysis of Bouncebacks
Our retrospective analysis included 504 transfers during 464 admissions of 486 patients from July 1, 2012 to October 27, 2014 (Fig. 2). Of these patients, 34 (6.7%; 95% CI 4–9%) transfers resulted in unplanned transfers to units capable of higher level care within 48 h of transfer. Age, sex, ICU length of stay, and both groups had similar rates of transfers between 6 pm and 6 am. Patients transferred between 6 pm and 6 am were not more likely to bounce back, and bounces were not more likely to happen after 6 pm. Hospital length of stay was increased among patients who bounced back but failed to meet significance (p = 0.06; Table 1). Hospital disposition was also similar between the groups (chi-squared p = 0.31). The most common reasons for bounceback were respiratory failure/hypoxia, sepsis/hypotension, provider desire for closer nursing monitoring, and cardiac arrhythmias (Fig. 2b).
Retrospective chart review results. a Case ascertainment: Patients transferred from the neuro-ICU from July 1, 2012 to October 27, 2014 were reviewed. There were 504 total transfer events of 486 patients that were analyzed among which there were 34 bounceback events of 31 patients. b Reasons for bounceback: Chart review was performed by an experienced clinician (DC) for each of the 34 bounceback events, and the primary reason for bounceback was determined in each case. EMR electronic medical record, GI bleed gastrointestinal bleed, HTN hypertension, ICU intensive care unit, LOS length of stay
Part 2: Intervention
During the intervention, 225 transfers during 214 admissions occurred and 8 bouncebacks (3.6%) occurred (Fig. 3a). During the intervention age, sex, and ICU length of stay were similar between two groups. Total hospital length of stay was higher among the patients who bounced back (p = 0.01; Table 2). A total of 128 out of 225 (57%) neuro-ICU transfers were labeled as high risk by the tool. One hundred percent of the bouncebacks that occurred during the study period were classified as high risk (8/8, sensitivity 100%, specificity 45%). Being high risk by the screening tool was associated with risk of bounceback (OR not calculable, p = 0.02). Two risk factors were independently associated with a risk of bounceback: aspiration risk (OR 6.9, 95% CI 1.6–30, p = 0.01) and cardiac arrhythmia in the neuro-ICU (OR 7.1, 95% CI 1.6–32, p = 0.01). Having more risk factors on the checklist increased the likelihood of bouncing back (p < 0.01). Although other items on the checklist were not independently associated with bouncebacks in the sample, the removal of any item decreased the sensitivity below 100%. The AUC of the checklist using one risk factor to define high-risk patients was 0.69. When increased to two risk factors, this AUC increased to 0.81, and a three risk factor cutoff subsequently decreased the AUC to 0.52. Neuro-ICU length of stay was similar in the retrospective analysis and the intervention period. Bounceback rate was 6.7% prior to the intervention, 4.0% in stage 1, 4.4% in stage 2, and 2.8% in stage 3 of the intervention (p for trend = 0.09; Fig. 3c). During the pilot, seven of the bouncebacks were stroke patients, a diagnosis that made bounceback more likely (p = 0.017).
Quality improvement pilot. a Case ascertainment: Pilot was implemented from July 1, 2015 to April 1, 2016. There were 225 eligible neuro-ICU transfers of 212 patients of which there were eight bouncebacks. b Reasons for bounceback: Chart review was performed by an experienced clinician (DC) for each of the eight bounceback events, and the primary reason for bounceback was determined in each case. c Rates of bounceback: Pre-intervention bounceback rate was 6.7%. The total bounceback rate for the intervention was 3.6% (p = 0.37). Stage 1 bounceback rate was 4.0%. Stage 2 bounceback rate was 4.5%. Stage 3 bounceback rate was 2.8%. p for trend = 0.086. LOS length of stay, neuro-ICU neurointensive care unit
Compliance with the intervention was high. Risk status was integrated into physician handoffs 79% of the time. Completed checklists were able to be analyzed in 60% of cases. Mean time from transfer to assessment by the receiving floor physicians was 18 min. Mean time to assessment by a respiratory therapist was 75 min. Seventy-six percent of high-risk transfers continued to be followed by respiratory therapists after their initial assessment. A total of 24 out of 29 (83%) neuro-ICU physicians thought the checklist was the appropriate length. A total of 14 out of 14 (100%) floor residents and 3/4 (75%) respiratory therapists thought that their time goals were sufficient. A total of 27 out of 41 (66%) respondents felt that the intervention improved patient care (Fig. 3a). Among these respondents, nurses were the least likely to believe that the intervention improved patient care 7/13 (54%); however, the intervention changed their workflow the least. A total of 35 out of 41 (85%) thought that it should become standard of care as is or with minor modification (see Supplemental Data). The most common suggestions for process improvement cited in the survey was streamlining the role of the neuro-ICU provider and limiting the role of respiratory therapists to patients who were deemed “high risk” due to respiratory-associated risk factors. There was no difference in age, sex, or ICU length of stay, between the retrospective patient group and during the quality improvement pilot although overall hospital length of stay was less during the quality improvement pilot (p = 0.03).
Discussion
ICU bouncebacks likely result from a combination of processes that are patient-specific, disease-specific, and healthcare system-specific [32, 33]. Some bouncebacks are likely unpreventable and represent new clinical events or unavoidable progression of disease [32]; consequently, their worth as a quality care metric has been questioned [7, 22, 33]. However, many bouncebacks are likely preventable due to premature discharge from ICUs, insufficient care after transfer, or failures in communication during critical handoff periods [27, 34,35,36]. An optimal bounceback rate is unclear, but is likely not zero percent given the inevitability of new clinical events and that managing patients in an intensive care setting for longer would cause practical problems with bed flow, lead higher costs, and lead to suboptimal overall resource utilization.
The most common factors cited as leading to unplanned ICU readmissions are respiratory distress/hypoxia, cardiac arrhythmias, and sepsis [3,4,5, 10,11,12,13, 37, 38]. Other risk factors that are cited less commonly include recent fever, neurological diagnosis, upper gastrointestinal bleeding, and older age [7, 13, 39]; however, these risk factors are largely derived from medical, surgical, and combined ICUs instead of those with exclusively neuro-ICU patients cared for in dedicated units. In the study that focused on neurologic patients, similarly non-neuromuscular respiratory failure and sepsis were the leading causes of bouncebacks [9]. Aside from clinical diagnoses, a variety of both single-item values, including hematocrit, respiratory rate, and PaCO2, have been reported to be associated with ICU readmission [7, 38, 39] and several composite scores of acuity and illness including APACHE II and APS have been examined as possible predictors [7, 14,15,16,17]. Overnight transfers have been thought to potentially relate to a higher risk of bounceback, but studies have been mixed [10, 18, 19] as have studies of ICU stress and acuity [11, 20].
Several strategies at improving care for patients around the time of transfer have been utilized. Checklists focusing on structured reporting of a standard set of information have been utilized in several studies and settings [12, 31, 40,41,42] but have come under fire for adding burden to providers with sometimes unclear utility [43, 44]. ICU outreach programs in some setting have been shown to aid in transitions both to and from the ICU, aiding in communication and in some studies, decreasing mortality [24,25,26,27, 45, 46]. Lastly, integrating of several services around the time of transfer is another potentially useful strategy [28, 29].
At our urban academic tertiary care center, the bounceback rate for neurology patients over a nearly 28-month period was 6.7% after removing patients meeting exclusionary criteria; this was similar to other ICUs in our health system and other studies [9, 12, 13, 21,22,23, 38]. While those patients had a myriad of primary neurologic diagnoses, the reasons for their bouncebacks were largely medical. Several of these issues were common sequelae of their primary neurologic diagnoses or frequently co-occur, which further serves to emphasize the necessity of multidisciplinary care for neurologic patients. Similar to previous studies, respiratory distress and hypoxia, sepsis/hypotension, and cardiac arrhythmias drove the majority of the bouncebacks rather than primary neurological events such as recurrent stroke or seizure. Our study did not show a difference in post-hospitalization disposition as has been demonstrated in prior studies, and we only saw a significant increase in hospital length of stay in bounceback cases during the pilot project. It is possible that the sample size was not large enough to detect a difference given the low number of bouncebacks that occurred in the retrospective portion of the study.
Based on the results of our retrospective analysis, as well as previous studies, we designed a simple checklist to help identify patients who may be at high risk of decompensation within 48 h of transfer. The checklist was easy to use, quick to complete, and served as a highly visible reminder of the patients’ risk factors. After extensive stakeholder analysis and contextual inquiry, we designed a multidisciplinary transfer process that included physicians, nurses, and respiratory therapists and utilized technology to help augment communication and evaluation of patients felt to be at “high risk” for bounceback. The intervention was implemented in stages, which allowed for familiarization and sequential workflow refinements prior to the addition of other care providers. Multiple rounds of provider education aided with initial familiarization and refinements to the checklist layout and workflow occurred throughout the intervention.
Our tool was able to identify patients who were at high risk for bounceback, and two factors, subjective aspiration risk and cardiac arrhythmia in the ICU, were both independent predictors of bouncebacks. Analysis indicated that this checklist performed would have performed optimally if the presence of two risk factors instead of one was used to define a transferring patient as high risk. This will be a consideration moving forward weighing more efficient resource utilization against decreased sensitivity of the tool. Interestingly during the pilot, stroke patients were more likely to bounceback. This may indicate that our intervention was more effective for patients with non-stroke diagnoses and also highlights that this population may benefit from targeted interventions in the future. While there was a decrease in the incidence of bouncebacks between our retrospective analysis and during the pilot, this did not reach statistical significance. Stage 3 of the intervention, when all departments and technological changes were in place, had the lowest occurrence of bouncebacks. Lack of significance is likely due to low power owing to a low overall event rate.
ICU length of stay was not increased during the pilot, indicating that providers did not keep patients in the ICU for a longer period of time based on the intervention. Overall, compliance with the intervention was high and providers felt that it improved patient care and should be continued. The most common suggestions for future improvements included streamlining the ICU physicians’ portion of the documentation and limiting respiratory therapists’ involvement to patients with exclusively respiratory-associated risk factors.
There are several limitations to this study. We did not include neurosurgical patients in this initial pilot because the risk factors associated with neurosurgical patients were likely to be significantly different from a neuromedical population. Furthermore, the workflow of the neurosurgical ICU teams at our institution differs significantly from the neuromedical teams which would have required significant alterations in the quality improvement pilot. However, after the results of this pilot, our neurosurgical department has recently adopted their own version of this intervention which is currently in use. In our retrospective analysis, we did not compare the rates of risk factors against patients who did not bounceback, but rather used the commonly cited reasons for bounceback in those patients who did as well as factors cited in previous studies [3,4,5, 9,10,11,12]. NeuroICU bouncebacks occurred at a low rate in both the retrospective and prospective portions of our studies. There was not a significantly decreased risk of bounceback during the intervention, and continued data collection is necessary. There was data loss that was attributable to the physical loss of bounceback sheets; while this loss did not affect analysis of risk factors or bounceback rates, it may have affected analysis of compliance with pilot procedures and time to assessment goals. However, we believe the missing data to be non-differentially lost at random. The significance of bouncebacks from a floor to a step-down unit is also unclear as this population has not been studied elsewhere. Lastly, it is not clear whether this intervention performed at a single center is amenable to similar approach in other neuro-ICUs. A cornerstone of quality improvement work is the use of contextual inquiry in local environments to help design procedures and initiatives. While this work may not be immediately generalizable, we believe that this approach, similarly applied, may lead to center-specific solutions that aid in improving care for this population during a vulnerable transition.
Conclusion
In conclusion, we utilized local information from our experience with nearly two and a half years of neuro-ICU bouncebacks and prior studies to develop a simple one-page checklist to identify risk factors for neurologic patients who were at high risk for bounceback after transfer from a neuro-ICU. After stakeholder analysis and gathering input from physicians, nursing, and respiratory therapy, we designed an intervention that centered on improved multidisciplinary communication surrounding those risk factors and early assessment after transfer. After a 9-month pilot period with a staged roll out, providers felt that care was improved and that the new communication paradigm should be continued. During the pilot, bounceback rates trended downwards. This simple tool was able to adequately identify “high-risk” patients during the pilot study and cardiac arrhythmia in the ICU and aspiration risk were both independent risk factors associated with bounceback. An augmented multidisciplinary handoff procedure for these high-risk patients may be helpful in aiding the transfer process from the neuro-ICU, and increased attention especially to neurologically associated medical needs by physicians, nurses, and respiratory therapists may be useful in improving care for this population.
References
SCCM Quality Indicators Committee. Candidate critical care quality indicators. Anaheim: Society of Critical Care Medicine; 1995.
Rhodes A, Moreno RP, Azoulay E, Capuzzo M, Chiche JD, Eddleston J, et al. Prospectively defined indicators to improve the safety and quality of care for critically ill patients: a report from the task force on safety and quality of the european society of intensive care medicine (ESICM). Intensive Care Med. 2012;38(4):598–605.
Rosenberg AL, Watts C. Patients readmitted to ICUs: a systematic review of risk factors and outcomes. Chest. 2000;118(2):492–502.
Rosenberg AL, Hofer TP, Hayward RA, Strachan C, Watts CM. Who bounces back? Physiologic and other predictors of intensive care unit readmission. Crit Care Med. 2001;29(3):511–8.
Kramer AA, Higgins TL, Zimmerman JE. Intensive care unit readmissions in U.S. hospitals: patient characteristics, risk factors, and outcomes. Crit Care Med. 2012;40(1):3–10.
Alban RF, Nisim AA, Ho J, Nishi GK, Shabot MM. Readmission to surgical intensive care increases severity-adjusted patient mortality. J Trauma. 2006;60(5):1027–31.
Cooper GS, Sirio CA, Rotondi AJ, Shepardson LB, Rosenthal GE. Are readmissions to the intensive care unit a useful measure of hospital performance? Med Care. 1999;37(4):399–408.
Priestap FA, Martin CM. Impact of intensive care unit discharge time on patient outcome. Crit Care Med. 2006;34(12):2946–51.
Gold CA, Mayer SA, Lennihan L, Claassen J, Willey JZ. Unplanned transfers from hospital wards to the neurological intensive care unit. Neurocrit Care. 2015;23(2):159–65.
Duke GJ, Green JV, Briedis JH. Night-shift discharge from intensive care unit increases the mortality-risk of ICU survivors. Anaesth Intensive Care. 2004;32(5):697–701.
Gajic O, Malinchoc M, Comfere TB, Harris MR, Achouiti A, Yilmaz M, et al. The Stability and workload index for transfer score predicts unplanned intensive care unit patient readmission: initial development and validation. Crit Care Med. 2008;36(3):676–82.
Brown SE, Ratcliffe SJ, Halpern SD. An empirical derivation of the optimal time interval for defining ICU readmissions. Med Care. 2013;51(8):706–14.
Chen LM, Martin CM, Keenan SP, Sibbald WJ. Patients readmitted to the intensive care unit during the same hospitalization: clinical features and outcomes. Crit Care Med. 1998;26(11):1834–41.
Chung DA, Sharples LD, Nashef SA. A case-control analysis of readmissions to the cardiac surgical intensive care unit. Eur J Cardio-Thorac Surg. 2002;22(2):282–6.
Frost SA, Alexandrou E, Bogdanovski T, Salamonson Y, Davidson PM, Parr MJ, et al. Severity of illness and risk of readmission to intensive care: a meta-analysis. Resuscitation. 2009;80(5):505–10.
Lee H, Lim CW, Hong HP, Ju JW, Jeon YT, Hwang JW, et al. Efficacy of the APACHE II score at ICU discharge in predicting post-ICU mortality and ICU readmission in critically ill surgical patients. Anaesth Intensive Care. 2015;43(2):175–86.
Wong EG, Parker AM, Leung DG, Brigham EP, Arbaje AI. Association of severity of illness and intensive care unit readmission: a systematic review. Heart Lung J Crit Care. 2016;45(1):3–9.
Hanane T, Keegan MT, Seferian EG, Gajic O, Afessa B. The association between nighttime transfer from the intensive care unit and patient outcome. Crit Care Med. 2008;36(8):2232–7.
Tobin AE, Santamaria JD. After-hours discharges from intensive care are associated with increased mortality. Med J Aust. 2006;184(7):334–7.
Chrusch CA, Olafson KP, McMillan PM, Roberts DE, Gray PR. High occupancy increases the risk of early death or readmission after transfer from intensive care. Crit Care Med. 2009;37(10):2753–8.
Fakhry SM, Leon S, Derderian C, Al-Harakeh H, Ferguson PL. Intensive care unit bounce back in trauma patients: an analysis of unplanned returns to the intensive care unit. J Trauma Acute Care Surg. 2013;74(6):1528–33.
Siddiqui S. Patients readmitted to the intensive care unit: can they be prevented? Int Arch Med. 2013;6(1):18.
van Sluisveld N, Bakhshi-Raiez F, de Keizer N, Holman R, Wester G, Wollersheim H, et al. Variation in rates of ICU readmissions and post-ICU in-hospital mortality and their association with ICU discharge practices. BMC Health Serv Res. 2017;17(1):281.
Franklin C, Mathew J. Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Crit Care Med. 1994;22(2):244–7.
Garcea G, Thomasset S, McClelland L, Leslie A, Berry DP. Impact of a critical care outreach team on critical care readmissions and mortality. Acta Anaesthesiol Scand. 2004;48(9):1096–100.
Esmonde L, McDonnell A, Ball C, Waskett C, Morgan R, Rashidian A, et al. Investigating the effectiveness of critical care outreach services: a systematic review. Intensive Care Med. 2006;32(11):1713–21.
van Sluisveld N, Hesselink G, van der Hoeven JG, Westert G, Wollersheim H, Zegers M. Improving clinical handover between intensive care unit and general ward professionals at intensive care unit discharge. Intensive Care Med. 2015;41(4):589–604.
Coon EA, Kramer NM, Fabris RR, Burkholder DB, Klaas JP, Graff-Radford J, et al. Structured handoff checklists improve clinical measures in patients discharged from the neurointensive care unit. Neurol Clin Pract. 2015;5(1):42–9.
Wibrandt I, Lippert A. Improving patient safety in handover from intensive care unit to general ward: a systematic review. J Patient Saf. 2017. (Epub 2017/04/30).
van Sluisveld N, Oerlemans A, Westert G, van der Hoeven JG, Wollersheim H, Zegers M. Barriers and facilitators to improve safety and efficiency of the ICU discharge process: a mixed methods study. BMC Health Serv Res. 2017;17(1):251.
Hoffman RL, Saucier J, Dasani S, Collins T, Holena DN, Fitzpatrick M, et al. Development and implementation of a risk identification tool to facilitate critical care transitions for high-risk surgical patients. Int J Qual Health Care. 2017;1–8. (Epub 2017/04/04).
Al-Jaghbeer MJ, Tekwani SS, Gunn SR, Kahn JM. Incidence and etiology of potentially preventable ICU readmissions. Crit Care Med. 2016;44(9):1704–9.
Brown SE, Ratcliffe SJ, Halpern SD. Assessing the utility of ICU readmissions as a quality metric: an analysis of changes mediated by residency work-hour reforms. Chest. 2015;147(3):626–36.
Baigelman W, Katz R, Geary G. Patient readmission to critical care units during the same hospitalization at a community teaching hospital. Intensive Care Med. 1983;9(5):253–6.
Colvin MO, Eisen LA, Gong MN. Improving the patient handoff process in the intensive care unit: keys to reducing errors and improving outcomes. Semin Respir Crit Care Med. 2016;37(1):96–106.
Abraham J, Nguyen V, Almoosa KF, Patel B, Patel VL. Falling through the cracks: information breakdowns in critical care handoff communication. AMIA Annu Symp Proc AMIA Symp. 2011;2011:28–37.
Kirby EG, Durbin C. Establishment of a respiratory assessment team is associated with decreased mortality in patients re-admitted to the ICU. Respir Care. 1996;41(10):903–7.
Durbin CG Jr, Kopel RF. A case-control study of patients readmitted to the intensive care unit. Crit Care Med. 1993;21(10):1547–53.
Snow N, Bergin KT, Horrigan TP. Readmission of patients to the surgical intensive care unit: patient profiles and possibilities for prevention. Crit Care Med. 1985;13(11):961–4.
Agarwal HS, Saville BR, Slayton JM, Donahue BS, Daves S, Christian KG, et al. Standardized postoperative handover process improves outcomes in the intensive care unit: a model for operational sustainability and improved team performance. Crit Care Med. 2012;40(7):2109–15.
Lane-Fall MB, Beidas RS, Pascual JL, Collard ML, Peifer HG, Chavez TJ, et al. Handoffs and transitions in critical care (HATRICC): protocol for a mixed methods study of operating room to intensive care unit handoffs. BMC Surg. 2014;14:96.
Halvorson S, Wheeler B, Willis M, Watters J, Eastman J, O’Donnell R, et al. A multidisciplinary initiative to standardize intensive care to acute care transitions. Int J Qual Health. 2016;28(5):615–25.
Smischney NJ, Cawcutt KA, O’Horo JC, Sevilla Berrios RA, Whalen FX. Intensive care unit readmission prevention checklist: is it worth the effort? J Eval Clin Pract. 2014;20(4):348–51.
Ko HC, Turner TJ, Finnigan MA. Systematic review of safety checklists for use by medical care teams in acute hospital settings—limited evidence of effectiveness. BMC Health Serv Res. 2011;11:211.
Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet (London, England). 2005;365(9477):2091–7.
Leary T, Ridley S. Impact of an Outreach team on re-admissions to a critical care unit. Anaesthesia. 2003;58(4):328–32.
Acknowledgements
The authors would like to acknowledge the contributions and support of Bethany Young RN, Lindsay Draham RN, Elizabeth Howe RN, Robert Bayer RT, Thomas Szybowski RT, Alex Rineer RN, Phyllis Dubendorf RN, and Heidi Lewis RN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Coughlin has nothing to disclose. Dr. Kasner reports grants from WL Gore, personal fees from Boehringer Ingelhim, personal fees from Bristol Meyers Squibb, personal fees from Bayer, grants from Acorda, personal fees from Medtronic, personal fees from UpToDate, grants from SanBio, and grants from AZ Therapies, outside the submitted work. Dr. Kumar has nothing to disclose. Dr. Patel has nothing to disclose. Dr. Hoffman has nothing to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Regarding the retrospective portion of this project, for this type of study formal consent is not required. Regarding the prospective quality improvement portion of this project, it was approved by the University of Pennsylvania’s IRB as a quality improvement project and thus did not require formal consent of patients involved.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coughlin, D.G., Kumar, M.A., Patel, N.N. et al. Preventing Early Bouncebacks to the Neurointensive Care Unit: A Retrospective Analysis and Quality Improvement Pilot. Neurocrit Care 28, 175–183 (2018). https://doi.org/10.1007/s12028-017-0446-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-017-0446-z