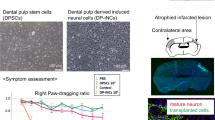
Abstract
Background
Transplantation of bone marrow or adipose-derived mesenchymal stem cells (MSCs) for various neurological disorders has yielded promising results in models of focal cerebral ischemia. Dental pulp stem cells (DPSCs) are a type of MSC. In serum-free culture, they can form neurospheres that contain nestin-positive neuronal progenitor cells. We hypothesized that transplantation of dental pulp-derived neurosphere cells would ameliorate outcomes of global cerebral ischemia, the pathophysiology of which is known to resist conventional treatments. We also hypothesized that transplantation of dental pulp-derived cells would provide some neuroprotection in this pathology due to the presence of DPSCs.
Methods
Using adult rats, ischemia was induced by two-vessel occlusion of both carotid arteries in combination with systemic hypotension. Allogeneic dental pulp cells from juvenile rats were cultured in advance in serum-free medium to obtain neurospheres. Dental pulp-derived neurosphere cells or dental pulp-derived cells were intravenously administered at 3 h after ischemic insult, with normal saline as a control. Animals were observed for 14 days after ischemia. Neurological outcome was assessed using the water-maze test and neuromotor test. Histological outcome was measured by counting the percentage of dead neurons in the hippocampal CA1 and CA3 regions.
Results
Transplantation of both dental pulp-derived neurosphere cells and dental pulp-derived cells significantly improved survival rate and water-maze test results. Neurosphere cell transplantation was related to significantly better neuromotor test and histological outcomes, as indicated by the reduced percentage of dead neurons in CA1.
Conclusions
Transplantation of dental pulp-derived neurosphere cells ameliorated outcomes of global cerebral ischemia. It was also demonstrated that dental pulp-derived cell administration provided some neuroprotection.




Similar content being viewed by others
References
Schneider A, Böttiger BW, Popp E. Cerebral resuscitation after cardiocirculatory arrest. Anesth Analg. 2009;108(3):971–9.
Crumrine RC, Bergstrand K, Cooper AT, Faison WL, Cooper BR. Lamotrigine protects hippocampal CA1 neurons from ischemic damage after cardiac arrest. Stroke. 1997;28(11):2230–7.
Xu K, Puchowicz MA, Lust WD, LaManna JC. Adenosine treatment delays postischemic hippocampal CA1 loss after cardiac arrest and resuscitation in rats. Brain Res. 2006;1071(1):208–17.
Lagina AT, Calo L, Deogracias M, Sanderson T, Kumar R, Wider J, et al. Combination therapy with insulin-like growth factor-1 and hypothermia synergistically improves outcome after transient global brain ischemia in the rat. Acad Emerg Med. 2013;20(4):344–51.
Noppens RR, Kofler J, Grafe MR, Hurn PD, Traystman RJ. Estradiol after cardiac arrest and cardiopulmonary resuscitation is neuroprotective and mediated through estrogen receptor-β. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2009;29(2):277–86.
Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: post-cardiac arrest care 2015 american heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S465–82.
Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2016;(2). Article No. CD004128. doi:10.1002/14651858.CD004128.pub4.
Zheng W, Honmou O, Miyata K, Harada K, Suzuki J, Liu H, et al. Therapeutic benefits of human mesenchymal stem cells derived from bone marrow after global cerebral ischemia. Brain Res. 2010;15(1310):8–16.
Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105(38):14638–43.
Chung TN, Kim JH, Choi BY, Chung SP, Kwon SW, Suh SW. Adipose-derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood-brain barrier disruption and endothelial damage. Stem Cells Transl Med. 2015;4(2):178–85.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–5.
Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122(1):80–90.
Tamaki Y, Nakahara T, Ishikawa H, Sato S. In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology. 2012;101(2):121–32.
Sugiyama M, Iohara K, Wakita H, Hattori H, Ueda M, Matsushita K, et al. Dental pulp-derived CD31 −/CD146 − side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. 2011;17(9–10):1303–11.
Inoue T, Sugiyama M, Hattori H, Wakita H, Wakabayashi T, Ueda M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. 2013;19(1–2):24–9.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci. 2003;100(10):5807–12.
Poltavtseva RA, Nikonova YA, Selezneva II, Yaroslavtseva AK, Stepanenko VN, Esipov RS, et al. Mesenchymal stem cells from human dental pulp: isolation, characteristics, and potencies of targeted differentiation. Bull Exp Biol Med. 2014;158(1):164–9.
Sasaki R, Aoki S, Yamato M, Uchiyama H, Wada K, Okano T, et al. Neurosphere generation from dental pulp of adult rat incisor. Eur J Neurosci. 2008;27(3):538–48.
Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci. 2010;11(3):176–87.
Capone C, Frigerio S, Fumagalli S, Gelati M, Principato M-C, Storini C, et al. Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. Plos One. 2007;2(4):e373.
Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238(1):120–32.
Miura Y, Kanazawa K, Nasu I. Preischemic Administration of sevoflurane does not exert dose-dependent effects on the outcome of severe forebrain ischemia in rats. J Neurosurg Anesthesiol. 2015;27(3):216–21.
Pacey L, Stead S, Gleave J, Tomczyk K, Doering L. Neural stem cell culture: neurosphere generation, microscopical analysis and cryopreservation. Protoc Exch [Internet]. 2006 Aug 25; http://www.nature.com/protocolexchange/protocols/77.
Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60.
Miura Y, Grocott HP, Bart RD, Pearlstein RD, Dexter F, Warner DS. Differential effects of anesthetic agents on outcome from near-complete but not incomplete global ischemia in the rat. Anesthesiology. 1998;89(2):391–400.
Mangus DB, Huang L, Applegate PM, Gatling JW, Zhang J, Applegate RL. A systematic review of neuroprotective strategies after cardiac arrest: from bench to bedside (Part I—protection via specific pathways). Med Gas Res. 2014;1(4):9.
Huang L, Applegate PM, Gatling JW, Mangus DB, Zhang J, Applegate RL. A systematic review of neuroprotective strategies after cardiac arrest: from bench to bedside (part II-comprehensive protection). Med Gas Res. 2014;20(4):10.
Silverman MG, Scirica BM. Cardiac arrest and therapeutic hypothermia. Trends Cardiovasc Med. 2016;26(4):337–44.
Hao L, Zou Z, Tian H, Zhang Y, Zhou H, Liu L. Stem cell-based therapies for ischemic stroke. Biomed Res Int. 2014;2014:468748.
Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134(6):1790–807.
Kawaguchi M, Kimbro JR, Drummond JC, Cole DJ, Kelly PJ, Patel PM. Isoflurane delays but does not prevent cerebral infarction in rats subjected to focal ischemia. Anesthesiology. 2000;92(5):1335–42.
Nasu I, Yokoo N, Takaoka S, Takata K, Hoshikawa T, Okada M, et al. The dose-dependent effects of isoflurane on outcome from severe forebrain ischemia in the rat. Anesth Analg. 2006;103(2):413–8.
Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. IV infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136(1):161–9.
Liu L, Eckert MA, Riazifar H, Kang D-K, Agalliu D, Zhao W. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013;2013:435093.
Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456(3):120–3.
Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73(6):778–86.
Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, et al. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38(7):2150–6.
Tamaoki N, Takahashi K, Tanaka T, Ichisaka T, Aoki H, Takeda-Kawaguchi T, et al. Dental pulp cells for induced pluripotent stem cell banking. J Dent Res. 2010;89(8):773–8.
Chun SY, Soker S, Jang Y-J, Kwon TG, Yoo ES. Differentiation of human dental pulp stem cells into dopaminergic neuron-like cells in vitro. J Korean Med Sci. 2016;31(2):171–7.
Funding
This study was supported by the “High-Tech Research Center” Project for Private Universities, with a matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Kumasaka, A., Kanazawa, K., Ohke, H. et al. Post-ischemic Intravenous Administration of Allogeneic Dental Pulp-Derived Neurosphere Cells Ameliorated Outcomes of Severe Forebrain Ischemia in Rats. Neurocrit Care 26, 133–142 (2017). https://doi.org/10.1007/s12028-016-0304-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0304-4