Abstract
Introduction
There are few predictors of acute lung injury (ALI) or the acute respiratory distress syndrome (ARDS) after subarachnoid hemorrhage (SAH). We hypothesized that cardiac troponin I, which is associated with cardiovascular morbidity, would also predict ALI.
Methods
We prospectively enrolled 171 consecutive patients with SAH. Troponin was routinely measured on admission and the next day and subsequently if abnormal. We prospectively recorded the maximum troponin, in-hospital events, and clinical endpoints. ALI and ARDS were defined by standard criteria.
Results
Acute lung injury was found in 10 patients (6%), ARDS in an additional 14 (8%), and pulmonary edema without lung injury in 9 (5%). Maximum troponin was different in patients without lung injury or pulmonary edema (0.03 [0.02–0.12] mcg/l), ALI (0.17 [0.04–1.4]), or ARDS (0.31 [0.9–1.8], P < 0.001). In ROC analysis, a cutoff of 0.04 mcg/l had 91% sensitivity and 42% specificity for ALI or ARDS (AUC = 0.75, P < 0.001). Troponin was associated with ALI or ARDS after accounting for neurologic grade in multivariate models without further contribution from pneumonia, packed red cell transfusion, gender, tobacco use, coronary artery disease, vasospasm, depressed ejection fraction on echocardiography, or CT grade. Lung injury was associated with worse functional outcome at 14 days, but not at 28 days or 3 months.
Conclusion
Troponin I is associated with the development of ALI after SAH.
Similar content being viewed by others
Introduction
Subarachnoid hemorrhage (SAH) is a neurologic emergency with high morbidity and mortality. While the most important predictor of outcome is neurologic grade on admission, medical complications are associated with worse outcomes in patients with SAH [1]. Detectable lung dysfunction has been reported in a majority of patients with SAH [2]. Lung injury may occur with equal plasma and endobronchial fluid protein content, implying increased pulmonary capillary permeability as the culprit [3]. Some have implicated neurogenic pulmonary edema (PED) [4] as a common cause of lung injury after SAH, while others have disputed a neurally mediated cause [5] or found a hemodynamic explanation [6].
Cardiac troponin I is often elevated in the first few days after SAH, and is probably related to a catecholamine and inflammatory surge that accompanies aneurysm rupture. Mild elevations in troponin (between 0.04 and 2 mcg/l) have been associated with hypotension requiring vasopressors [7], a depressed ejection fraction on echocardiography [8], regional wall motion abnormalities [9], and cardiovascular mortality after SAH [10]. This syndrome is generally termed neurogenic stunned myocardium or myocardial stress cardiomyopathy [8]. A similar process may underlie neurogenic PED [11–13], so we hypothesized that troponin would predict acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) after SAH.
Materials and Methods
Study Population. We prospectively enrolled consecutive patients with SAH, diagnosed by the admission computed tomography (CT) scan or by xanthochromia of the cerebrospinal fluid if the CT was non-diagnostic. Patients admitted within 14 days of spontaneous aneurysmal and cryptogenic SAH were included; patients with trauma or other structural lesions (e.g., vascular malformations) were excluded. All data were prospectively recorded. Admission CT scans were graded with the Columbia CT scale, which accounts for thick subarachnoid clot and intraventricular hemorrhage [14] from grade 1 (thin clot without intraventricular hemorrhage) to 4 (thick clot with bilateral intraventricular hemorrhage).
Clinical Management. Diagnostic catheter or CT angiography and aneurysm obliteration with surgical clipping or endovascular coiling were performed as soon as possible. Enteral nimodipine [15] was given unless the systolic blood pressure was <120 mmHg. We maintained central venous pressure ≥5 mmHg [16] as measured with a central venous catheter. Transcranial Doppler sonography was performed daily. Vasospasm was defined as any mean transcranial Doppler velocity >120 cm/s or clinical vasospasm. (We consider both definitions because comatose patients may not have a change in the neurologic exam.) Clinical vasospasm was treated with hyperdynamic therapy and a goal systolic blood pressure of 180–200 mmHg or resolution of clinical symptoms, and intra-arterial vasodilators and angioplasty if clinically appropriate. We transfused leukoreduced packed red blood cells (PRBCs) for hemoglobin <10 g/dl [17, 18]. When a PRBC transfusion was given, we prospectively recorded the acute onset of dyspnea, hypoxemia, respiratory failure, or new infiltrates on chest radiography.
Ventilatory support management was at the discretion of the critical care team in concert with registered respiratory therapists. Patients with ALI or ARDS were managed with reduced tidal volumes and minimized plateau pressures [19]. Our practice is to start with SIMV mode and positive end-expiratory pressure of 5 cm water. We switch to a titrated level of pressure support when and if the patient is able to initiate respirations, and wean the pressure support to 5 cm H2O as tolerated. Ventilatory support is removed when the patient meets criteria on a standardized checklist for oxygenation, respirations, bronchial hygiene, and alertness.
Clinical variables were prospectively collected. We recorded baseline demographic and past medical history data onto forms. Neurological status on admission was assessed with the World Federation of Neurological Surgeons (WFNS) scale, derived from the Glasgow Coma Scale (GCS) [20]. The WFNS is graded as 1 (GCS 15), 2 (GCS 13–14 without motor deficit), 3 (GCS 13–14 with motor deficit), 4 (GCS 7–12), or 5 (GCS 3–6). We prospectively recorded the occurrence of bacteremia (any positive blood cultures, with the exception of one sample that was clinically judged to be a contaminant). Pneumonia was diagnosed by criteria by the US Centers for Disease Control [21] and the date of appearance was recorded.
Diagnosis of acute lung injury. We prospectively evaluated chest radiographs (obtained daily in intubated patients and with any change in respiratory status in non-intubated patients) and the diagnostic arterial blood gas. Lung injury was diagnosed by consensus criteria [22] of PaO2/FiO2 <300 (ALI) or <200 (ARDS), new bilateral pulmonary infiltrates, and clinical absence of cardiac cause. Patients were classified as having only one type of lung injury. We classified radiographic PED as acute bilateral infiltrates on chest radiography with a PaO2/FiO2 ratio of ≥300.
Cardiac troponin I was routinely measured on admission and at the next day, and followed if abnormal (>0.04 mcg/l) or if the patient received vasopressors. We defined a depressed left ventricular ejection fraction on echocardiography as <55% on the official echocardiography interpretation.
Outcomes. At 14 days or discharge, whichever was first, a certified examiner recorded the NIH Stroke Scale and the modified Rankin Scale (mRS), a global disability and handicap scale ranging from 0 (no deficits) to 6 (dead). We obtained a follow-up Rankin at 28 days and 3 months with a standardized questionnaire [23].
Statistical analysis. Non-normally distributed variables are presented as median [inter-quartile range] and were compared with Mann–Whitney U (2 groups) or Kruskal–Wallis H (>2 groups) as appropriate. Continuous variables were compared with ANOVA and multiple comparisons were corrected with the Least Significant Differences technique. Categorical variables were analyzed with chi-squared. Troponin was categorized into quartiles (undetectable through 0.04, 0.05–0.5, >0.5–2, and >2 mcg/l) for logistic regression analysis because it was not normally distributed (skewed with a tail to the right). Quartiles of troponin, WFNS, and CT grade were classified as ordinal variables in logistic regression. Statistical calculations were made with standard commercial software (SPSS version 16, Chicago, IL).
The study was approved by the Institutional Review Board. Written informed consent was obtained from the patient or a legally authorized representative in all cases, except when the patient died in hospital or no representative could be located for an incapacitated patient, in which case the institutional review board approved collection of data in a registry without consent.
Results
Demographics and univariate associations with lung injury are in Table 1.
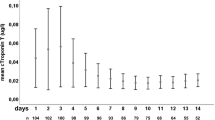
Troponin and lung injury. The ROC curve for troponin and ALI or ARDS is shown in Fig. 1. Troponin >0.04 mcg/l had a sensitivity of 0.92 and specificity of 0.42. The peak troponin occurred 1.5 [4.25–0] days before any PED on chest radiography, and 1 [3–0] days before the criteria for ALI or ARDS were met.
Transfusion related acute lung injury versus circulatory overload. Only one patient was prospectively recorded as having acute worsening in respiratory status with PRBC transfusion. This patient was a 77-year-old Caucasian woman with a WFNS grade 5 SAH. She received five units of PRBCs for a retroperitoneal hematoma 10 days after SAH. This episode was judged to be transfusion related circulatory overload rather than transfusion related ALI [24]. A 14-mm right vertebral artery aneurysm could not be obliterated endovascularly, and a balloon test occlusion of the parent vessel led to cerebral ischemia. A surgical occlusion and bypass were planned, but the patient was not stable enough to undergo the procedure despite full ICU care. She was declared dead by neurologic criteria from aneurysm rebleeding approximately 3 weeks later.
Infection. One patient with ARDS developed bacteremia 25 days later.
Logistic regression models for lung injury. In logistic regression WFNS grade, quartile of troponin and an interaction were associated with the development of ALI (Table 2). At low WFNS grade, troponin was strongly associated with lung injury, with less effect at higher neurologic grades. Pneumonia, PRBC transfusion, vasospasm, hyperdynamic therapy, gender, coronary artery disease, CT Score, a depressed ejection fraction, age, and tobacco use did not add to the model. We obtained similar results when we excluded patients with a depressed ejection fraction on echocardiography from the analysis.
Outcomes. Lung injury was associated with worse mRS at 14 days (P = 0.001). Of the 24 patients with ALI or ARDS, 21 were dependent, bed bound or dead (mRS 4–6). ALI or ARDS was not associated with mRS at 28 days or 3 months (P > 0.1).
Discussion
We found that troponin I was associated with ALI, and the association persisted in multivariate models. These results may help clinicians predict ALI in patients with SAH.
The troponin elevations we found were similar in magnitude to those previously described as having prognostic importance after SAH [10]. Temporary decreases in systolic function (consistent with neurogenic stunned myocardium) are well described after SAH [4, 8] and it is possible that very mild systolic dysfunction was not detected on routine echocardiography. Troponin elevations usually occur soon after SAH [10].
The cause of lung injury was not clear in most cases. Pneumonia was associated with lung injury, but explained less than half of cases. Sepsis did not occur before lung injury in our series. PRBC transfusion was associated with lung injury in univariate, but not multivariate analysis, and our only case of acute respiratory symptoms associated with transfusion was more likely to be circulatory overload when analyzed with a proposed algorithm [24]. The univariate associations we found were likely confounded by poor neurologic grade, and did not enter multivariate models.
Some investigators have stated that neurogenic PED is rare [2], while others have documented it more frequently [4]. The lack of a standardized definition of neurogenic PED makes it more difficult to ascertain the incidence. PED in our series was associated with modestly elevated troponin, generally preserved systolic function, and a normal PaO2/FiO2 ratio, and this might be a reasonable working definition of neurogenic PED. Elevated troponin has been associated with a variety of cardio-pulmonary complications after SAH [25].
We did not prospectively record ventilatory support settings, so we are unable to analyze how they might affect the development or outcome of lung injury after SAH, although we do so now. It is possible that troponin predicts patients at higher risk for ventilator induced lung injury after SAH and this possibility should be studied further.
We did not perform analysis of the protein content of endobronchial fluid [3, 26]. We had no data available on pulmonary artery catheter measurements in this patient population. Like most centers, our use of the pulmonary artery catheter in lung injury has declined [27] in line with negative clinical trial results [28], and we do not routinely use them in SAH. We did not keep track of daily fluid balance because the circulating blood volume in patients with SAH is resistant to hypervolemic therapy [16].
Lung injury may co-exist with a depressed ejection fraction that is not causally related. Results were similar when patients with a depressed ejection fraction were excluded, however, making stunned myocardium an unlikely explanation for our findings. Echocardiograms were obtained near the time of lung injury and generally showed normal systolic function, so we are unlikely to have missed stunned myocardium as a cause. PED is usually not related to a temporary decrease in systolic function [29].
Hyperdynamic therapy for vasospasm might lead to PED as a complication, although we found that neurologic grade and cardiac troponin I precluded the addition of vasospasm (by clinical or TCD criteria) to the statistical model. We found lung injury typically followed SAH by three to four days, somewhat earlier than a previously reported series [30]. We did not find an effect of timing of lung injury on outcome.
Lung injury was associated with more dependence at 14 days, but functional outcomes were not significantly different at 28 days and 3 months. This may relate to improved recognition and management through standardized guidelines and intensive care. Neurologic grade, age, and cerebral infarction remain the most important predictors of neurologic and functional outcome. Our results contrast with a larger, retrospective study that found lung injury in 27% of patients and an associated increase in mortality [31].
In summary, elevated troponin after SAH was associated with ALI. Further research should examine if troponin identifies patients at higher risk for lung injury related to ventilatory support and if lower volume, low pressure ventilatory support improves outcomes after neurologic injury.
References
Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–23. doi:10.1097/00003246-200612002-00426.
Gruber A, Reinprecht A, Görzer H, Fridrich P, Czech T, Illievich UM, et al. Pulmonary function and radiographic abnormalities related to neurological outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1998;88:28–37.
Fein I, Rackow E. Neurogenic pulmonary edema. Chest. 1982;81:318–20. doi:10.1378/chest.81.3.318.
Vespa PM, Bleck TP. Neurogenic pulmonary edema and other mechanisms of impaired oxygenation after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2004;2:157–70. doi:10.1385/NCC:1:2:157.
Sarnoff SJ, Sarnoff LC. Neurohemodynamics of pulmonary edema. II. The role of sympathetic pathways in the elevation of pulmonary and systemic vascular pressures following the intracisternal injection of fibrin. Circulation. 1952;6:51–62.
Carlson R, Schaeffer R, Michaels S, Weil M. Pulmonary edema following intracranial hemorrhage. Chest. 1979;75:731–4. doi:10.1378/chest.75.6.731.
de Chazal I, Parham WM III, Liopyris P, Wijdicks EF. Delayed cardiogenic shock and acute lung injury after aneurysmal subarachnoid hemorrhage. Anesth Analg. 2005;100:1147–9. doi:10.1213/01.ANE.0000147704.90285.2A.
Lee VH, Oh JK, Mulvagh SL, Wijdicks EFM. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006;5:243–9. doi:10.1385/NCC:5:3:243.
Kothavale A, Banki NM, Kopelnik L, Yarlagadda S, Lawton MT, Ko N, et al. Predictors of left ventricular regional wall motion abnormalities after subarachnoid hemorrhage. Neurocrit Care. 2006;4:199–205. doi:10.1385/NCC:4:3:199.
Naidech AM, Kreiter K, Janjua N, Ostapkovich N, Parra A, Commichau C, et al. Cardiac troponin elevation, cardiovascular mortality, and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851–6. doi:10.1161/CIRCULATIONAHA.105.533620.
Friedman JA, Pichelmann MA, Piepgras DG, McIver JI, Toussaint LG III, McClelland RL, et al. Pulmonary complications of aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:1025–32. doi:10.1227/01.NEU.0000058222.59289.F1.
Theodore L, Robin E. Speculations on neurogenic pulmonary edema. Am Rev Respir Dis. 1976;113:405–11.
Mayer SA, Fink ME, Homma S, Sherman D, LiMandri G, Lennihan L, et al. Cardiac injury associated with neurogenic pulmonary edema following subarachnoid hemorrhage. Neurology. 1994;44:815–20.
Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: The fisher scale revisited. Stroke. 2001;32:2012–20. doi:10.1161/hs0901.095677.
Dorhout Mees SM, Rinkel GJ, Fein VL, Algra A, van den Bergh WM, Vermeulen M, et al. Calcium antagonists for aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:514–5. doi:10.1161/STROKEAHA.107.496802.
Lennihan L, Mayer SA, Fink ME, Beckford A, Paik MC, Zhang H, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2000;31:383–91.
Naidech A, Jovanovic B, Wartenberg K, Parra A, Ostapkovich N, Connolly E, et al. Higher hemoglobin is associated with improved outcome after subarachnoid hemorrhage. Crit Care Med. 2007;35:2383–9. doi:10.1097/01.CCM.0000284516.17580.2C.
Naidech A, Drescher J, Ault M, Shaibani A, Batjer H, Alberts M. Higher hemoglobin is associated with less cerebral infarction, poor outcome and death after subarachnoid hemorrhage. Neurosurgery. 2006;59:775–80. doi:10.1227/01.NEU.0000232662.86771.A9.
The acute respiratory distress syndrome network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi:10.1056/NEJM200005043421801.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4. doi:10.1016/S0140-6736(74)91639-0.
Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall C, editor. Hospital epidemiology and infection control. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. p. 1659–702.
Bernard G, Artigas A, Brigham K, Carlet J, Falke K, Hudson L, et al. The american-european consensus conference on ards. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24.
Marotta CA, Banks JL. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–6. doi:10.1161/01.STR.0000258355.23810.c6.
Gajic O, Gropper MA, Hubmayr RD. Pulmonary edema after transfusion: how to differentiate transfusion-associated circulatory overload from transfusion-related acute lung injury. Crit Care Med. 2006;34(Suppl):S109–13. doi:10.1097/01.CCM.0000214311.56231.23.
Schuiling WJ, Dennesen PJW, Tans JTJ, Kingma LM, Algra A, Rinkel GJE. Troponin I in predicting cardiac or pulmonary complications and outcome in subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2005;76:1565–9. doi:10.1136/jnnp.2004.060913.
Smith WS, Matthay MA. Evidence for a hydrostatic mechanism in human neurogenic pulmonary edema. Chest. 1997;111:1326–33. doi:10.1378/chest.111.5.1326.
Wiener R, Welch H. Trends in the use the of pulmonary artery catheter in the United States, 1993–2004. JAMA. 2007;298:458–61. doi:10.1001/jama.298.4.423.
The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;254:2213–24.
Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17–22. doi:10.1056/NEJM200101043440103.
Kramer AH, Bleck TP, Dumon AS, Kassell NF, Olson C, Nathan B. Implications of early versus late bilateral pulmonary infiltrates in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2009;10:20–7. doi:10.1007/s12028-008-9137-0.
Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors and outcome. Crit Care Med. 2006;34:196–202. doi:10.1097/01.CCM.0000194540.44020.8E.
Acknowledgments
Dr. Naidech has received past grant funding from the Northwestern Memorial Foundation, the Neurocritical Care Society and NovoNordisk to perform a pilot clinical trial of goal hemoglobin in patients with subarachnoid hemorrhage. That trial is registered at www.stroketrials.org. Dr. Naidech has current received research support from Astellas Pharma US and Gaymar Inc., and past speaker fees from EKR Therapeutics. The other authors decline any conflicts related to this work. This work was departmentally funded.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naidech, A.M., Bassin, S.L., Garg, R.K. et al. Cardiac Troponin I and Acute Lung Injury After Subarachnoid Hemorrhage. Neurocrit Care 11, 177–182 (2009). https://doi.org/10.1007/s12028-009-9223-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-009-9223-y