Abstract
Microglia function as a vital constituent in the maintenance of brain homeostasis. Aberrant microglial activation, however, may contribute to neurodegenerative diseases. Lithospermic acid (LA) is a plant-derived polycyclic phenolic carboxylic acid isolated from Salvia miltiorrhiza. The present study investigated the potential effects of lithospermic acid on LPS-induced neuroinflammation in BV2 microglial cells and determined the mechanism of action of this compound. Cells were pre-treated with lithospermic acid for 1 h and incubated with LPS for 24 h. qPCR, immunofluorescence, and immunoblot assays were used to determine the expression of iNOS, COX2, NF-κB p65, and HSP90 expression. ELISA was employed to measure the production of pro-inflammatory cytokines. Lithospermic acid dramatically reduced LPS-stimulated cell migration and decreased NF-κB p65 nuclear translocation. Furthermore, lithospermic acid also markedly decreased the production of pro-inflammatory cytokines, including IL-6, IL-1β, and TNF-α in a dose-dependent manner. Additionally, lithospermic acid inhibited NO and PGE2 production in response to LPS, and it also inhibited the expression of iNOS and COX2 in a dose-dependent manner. Molecular docking and experimental verification have demonstrated that lithospermic acid inhibits the activity and expression of HSP90. Small interfering RNA knockdown of HSP90 expression, which abrogated LPS-induced inflammation. These findings suggest that the lithospermic acid targeting HSP90 attenuates LPS-induced inflammatory response via the NF-κB signalling pathway in BV2 microglial cells. Collectively, lithospermic acid may offer therapeutic benefits for neurodegenerative disorders associated with microglial activation and could serve as a potential inhibitor/agent for the treatment of neuroinflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia are the resident macrophages of the central nervous system (CNS) and comprise approximately 10% of the cell population. Functionally, they play a crucial role in the automatic defense response and the clearance of pathogenic factors in the brain [1]. However, microglia are a double-edged sword and can be either neuroprotective or neurotoxic. Under physiological conditions, microglia, which are immune cells, constitute the initial line of defense against pathogens [2]. They are responsible for immune surveillance and maintenance of CNS homeostasis [3]. Under pathological conditions, such as brain trauma or exposure to LPS, IFN-γ, or β-amyloid, microglia become activated and release neurotoxic and pro-inflammatory mediators, including reactive oxygen species (ROS), nitric oxide (NO), prostaglandin E2 (PGE2), and pro-inflammatory cytokines. In addition, prolonged microglia activation can lead to neuroinflammation, which ultimately causes neuronal damage and synaptic dysfunction [3]. In light of the heightened correlation between microglial activation and neuronal dysfunction, as well as aberrant protein aggregation in Alzheimer’s and Parkinson’s disease, the modulation of the inflammatory response of microglia is regarded as a pivotal strategy for the prevention of neurodegenerative disease development and progression [4].
In neurotoxicological and immunological research, the BV2 mouse microglial cell line displays morphological, functional, and phenotypic characteristics that are analogous to those observed in primary microglia. Therefore, BV2 microglial cells are frequently employed as a substitute for primary microglia in the investigation of diverse microglial responses and interactions in vitro. Lipopolysaccharide (LPS) is one of the most extensively researched immunostimulatory endotoxins, expressed on the outer membrane of gram-negative bacteria. It is frequently employed to model the pro-inflammatory and neurotoxic activation of microglia [5, 6]. LPS induces an excessive activation of BV2 microglial cells by binding to Toll-like receptor 4 (TLR4), which ultimately activates a multitude of downstream signal transduction pathways, including the nuclear factor nuclear factor kappa B (NF-кB) signalling pathway. Furthermore, BV2 cell activation leads to the upregulation of HSP90 (heat shock protein 90), which in turn regulates the NF-кB signalling pathway [7]. Furthermore, it has been demonstrated that LPS-stimulated BV2 microglial cells can induce oxidative stress by enhancing the production of reactive oxygen species (ROS). ROS modifies the gene expression of proinflammatory mediators by activating NF-кB transcription factors. These events permit active NF-кB to translocate into the nucleus, thereby initiating the transcription of a series of pro-inflammatory genes, including inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX2), interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-alpha (TNF-α) [8,9,10]. Once activated, NF-кB led to an exacerbation and amplification of neuroinflammatory responses, which were accompanied by an increase in the production of pro-inflammatory cytokines [11]. In reaction to inflammatory stimuli, microglial cells are promptly activated to engage in pathological responses, including migration to the affected regions and the subsequent release of various inflammatory molecules. Consequently, an elevated rate of cell migration can also be regarded as a characteristic of activated microglial cells [12]. Taken together, modulating the HSP90 expression and NF-кB signalling pathway activation represents a potential therapeutic strategy for the prevention and treatment of neuroinflammatory diseases.
Traditional Chinese Medicine (TCM) has a long history of being employed in the treatment of human diseases and maintaining health through “multi-component, multi-target synergy and multi-pathways.” The search for suitable anti-inflammatory agents has recently focused on plants used in TCM, as natural products offer a more reliable and effective treatment with fewer side effects for brain diseases than synthetic compounds. Danshen, the dried root and rhizome of Salvia miltiorrhiza, is a conventional Chinese herbal remedy employed for the management of cardiovascular disorders. Furthermore, it has been demonstrated to exert cerebrovascular effects, in addition to exhibiting antioxidant, anti-fibrotic, anti-inflammatory, and anti-cancer activities [13]. Lithospermic acid (LA) is a biologically active component isolated from aqueous extraction of Danshen [13]. Recent pharmacological studies showed that lithospermic acid has antibacterial, anti-apoptotic, and anti-inflammatory effects. Increasing research attention is being directed towards the impact of LA on inflammation and related diseases. Nevertheless, the anti-inflammatory effect of LA on LPS-stimulated BV2 microglial cells has yet to be documented. Herein, the purpose of our study was to investigate the anti-inflammatory effect and mechanism of LA on LPS-stimulated BV2 microglial cells.
Materials and methods
Reagents and antibodies
Lithospermic acid (#HY-N0823) was bought from MCE (Princeton, USA). LPS (#L4391) and Hoechst 33,342 (#B2261) were bought from Sigma. Cell counting kit-8 (C0048S), Griess reagent kit (S0021S), Nuclear and cytoplasmic protein extraction kit (P0027), Crystal violet staining solution (C0121), and ROS assay kit (S0033S) were obtained from Beyotime. Fetal bovine serum and DMEM medium were bought from Thermo Fisher (Carlsbad, United States). Specific antibodies against iNOS (#13,120), COX2 (#12,282), NF-кB p65 (#8242), GAPDH (#5174), Histone H3 (#4499), HSP90 (#4874), HSP70 (#4876), and β-Actin (#3700) were purchased from CST (Danvers, United States). The mouse ELISA kits for IL-1β (#EK0394), IL-6 (#EK0411), and TNF-α (#EK0527) were purchased from Boster Bio. PGE2 ELISA kit (#SEKM-0173) was acquired from Solarbio.
Cell line culture
Mouse cell line BV2 (CL-0493) was obtained from the Procell (Wuhan, China). The cells were grown in a 5% CO2 humidified incubator at 37 °C, with a complete DMEM medium (containing 10% FBS). Every 2–3 days, subculture is performed.
Cell viability assay
The viability of the cells was determined through the CCK-8 assay, in accordance with the protocol outlined by the manufacturer and previously detailed in the earlier report [14]. Briefly, BV2 cells in the log phase were prepared by seeding them in a 96-well plate (1 × 104/well) and cultured overnight. Prior to priming with LPS for 24 h, cells were pre-treated with lithospermic acid at varying concentrations for 1 h. Subsequently, a detection reagent was added to each well, followed by an additional incubation period of 30 min at 37 °C. To represent 100% cell survival, the cell viability of the control group was used. The absorbance was recorded and measured at 450 nm using a microplate reader (Bio-Rad, USA).
Intracellular ROS generation analysis
The levels of ROS were quantified using the DCFH-DA probe (Beyotime, S0033). BV2 cells were seeded in 24-well plates at 5 × 104 cells/well. After treatment, cells were loaded with a DCFH-DA probe (10 µM) for 30 min. In the existence of ROS, DCFH interacts with ROS, resulting in the emergence of the fluorescent DCF product that is confined within the cells. DCF fluorescence was captured by fluorescence microscopy.
Cytosolic and nuclear fractionation
BV2 cells were seeded in a 100 mm dish at a density of 2 × 106 cells/well and incubated overnight. NF-кB p65 translocation was measured by performing cell extraction and isolating nuclear and cytoplasmic proteins, following the instructions (Beyotime, P0027). For harvesting cells, cells were scraped and centrifuged at 1500 × g for 5 min. During extraction, cell pellets were re-suspended in 200 µl of extraction buffer A and incubated for 10 min on ice. Subsequently, the sample was added to extraction buffer B and vortexed for 30 s at 4 °C. Afterward, the supernatants of the cytosolic fraction were then collected at 12,000 × g for 5 min, and the pellets were re-suspended in buffer C. Centrifugation of the samples was performed at 12,000 × g for 5 min, followed by collection of the nuclear extract supernatants for analysis. The concentration of nuclear and cytoplasmic proteins was determined with the BCA assay from Beyotime (P0010), followed by analysis of expression through western blot.
Immunoblotting analysis
BV2 cells were seeded in 6-well plates at 2 × 105 cells/well. Cell lysates were obtained in accordance with the methodology previously described in the report [15]. The total protein was isolated by SDS-PAGE and subsequently transferred to PVDF membranes by electroporation. The membranes were incubated with blocking buffer and then with the specified primary antibodies at 4 °C overnight. The secondary antibodies were incubated for an additional hour. The bands were visualized with the use of a BeyoECL Star reagent (Beyotime, P0018) and imprinted on X-ray films (Beyotime, FF082). An imaging system, the FluorChem8000, was used to capture blot images. Analyzing gray values was done with AlphaEaseFC 4.0 software.
Immunofluorescence microscopy
The immunofluorescence analysis was carried out as described previously [14]. In brief, cells were seeded in glass-bottomed dishes (5 × 104 cells/well) and incubated overnight. After 24 h, cells were subjected to fixation, permeabilization, and blocking, followed by incubation with antibodies overnight. Incubation with secondary antibodies followed for another hour. Immunofluorescence images were captured with a microscope, the Zeiss Axio Observer D1.
Real-time PCR assay
RNA from cells was extracted by total RNA isolation Kit (Yeasen, No.19221ES50). RNA samples reverse transcribed into cDNA (Yeasen, No. 11141ES10) and PCR amplifying (Yeasen, No. 11184ES08). Primer sequences used in this study were obtained from PrimerBank and are listed as follows: mouse iNOS (5′-TCGATGCACAACTGGGTGAAC-3′; 5′-GGAGTGACGGCAAACATGACT-3′), COX2 (5′-TCAGGAAGCTCCTTATTTCCCTT-3′; 5′-TGCACTATGGTTACAAAAGCTGG-3′), IL-6 (5′-TCTATACCACTTCACAAGTCGGA-3′; 5′-GAATTGCCATTGCACAACTCTTT-3′), IL-1β (5′-TTCAGGCAGGCAGTATCACTC-3′; 5′-GAAGGTCCACGGGAAAGACAC-3′), TNF-α (5′-CGATCACCCCGAAGTTCAGTAG-3′; 5′-CAGGCGGTGCCTATGTCTC-3′), and GAPDH (5′-GGGGTCGTTGATGGCAACA-3′; 5′-AGGTCGGTGTGAACGGATTTG-3′). The expression of the target genes was normalized to GAPDH.
ELISA for the determination of cytokine levels
The levels of inflammatory cytokines and PGE2 in the culture supernatants were performed using commercially available ELISA kits. The procedure was according to the protocols. The absorbance was recorded and measured at 450 nm using a microplate reader (Bio-Rad, USA).
NO generation determination
The concentration of nitrite, which serves as an indicator for the production of NO, was quantified in the supernatants through the use of the Griess reagent (Beyotime, S0021S). A microplate reader was used to record and measure absorbance at 540 nm (Bio-Rad, USA). A standard curve was generated using sodium nitrite diluted in DMEM medium at concentrations of 0–100 µM.
Wound healing assay
Cells were cultured overnight to form a confluent monolayer. In order to evaluate cell migration, a sterile pipette tip (10 µl) was used to equally scratch the monolayer. After scratching, carefully rinse the cells with PBS to eliminate any detached cells. Then, proceed to culture the cells in a fresh medium containing LPS (1 µg/ml), with or without lithospermic acid for, 24 h. Phase contrast microscopy measured wound area changes at 0 and 24 h. The quantitative analysis of the migration rate of the cells within 24 h was measured using the ImageJ software.
Transwell migration assay
Cells were seeded into transwell chambers (1 × 104/well) and then the upper chambers were primed with LPS, with or without lithospermic acid (100 µM) for 24 h. After treatment, wipe the membrane with cotton to wipe off non-migrated cells, and the migrated cells were fixed with 4% paraformaldehyde for 15 min and stained with 0.1% crystal violet solution for 10 min. Under phase-contrast microscopy, the number of cells traversing the membrane was enumerated from five randomly selected fields of view for each experimental group.
Identification of lithospermic acid targets
The simplified molecular input line entry system (SMILES) representation of lithospermic acid, along with its PubChem CID: 6,441,498 was uploaded into the SwissTargetPrediction (http://swisstargetprediction.ch), a comprehensive database that based on similarity searching for each compound. The species was restricted to “Mus musculus.” All potential targets were retrieved for further validation. In parallel, the RNA-seq data for WT and LPS-primed BV2 cells were retrieved from the NCBI sequence read archive (SRA) database with the following accession number: SRR10298351, SRR10298352, SRR10298353, SRR10298356, SRR10298361, and SRR10298362. The raw reads were processed with TrimGalore for quality trimming and adapter removal and then aligned to the mouse genome (mm10). FeatureCount was applied to generate the gene level count and transcript per million (TPM) was used to quantify the gene expression value. DESeq2 was employed to detect the differentially expressed genes (DEGs) in LPS-primed BV2 cells [16]. Finally, potential target genes were determined by combining the predicted genes and DEGs.
Construction of protein–protein interaction (PPI) network
Potential target genes were collected for constructing the PPI network by the STRING plug-in embedded in Cytoscape. Briefly, the gene list from these potential target genes was imported through the “Network from public Databases” option with default parameters, except for the Data Source, which was limited to “STRING protein query,” and Species, which was limited to “Mus musculus.” Topological parameters of the network, including the number of nodes, the distributions of node degrees, and connected components were computed in the Cytoscape plugin Network Analyzer. Finally, visualization for genes in the PPI network was also implemented in the Cytoscape.
Reactome pathway enrichment and network analyses
Potential target genes were submitted to the DAVID online database [17]. Pathway terms with a p-value less than 0.05 from the Reactome functional enrichment were retrieved. EnrichmentMap, a network-based visualization method implemented as a plug-in in the Cytoscape [18], was then utilized to construct the pathway network as described previously. In the EnrichmentMap, pathways are depicted as circular nodes, and lines connect these nodes when they share a number of genes. The network was organized into clusters using the clusterMaker2 software. Each cluster within the network represents pathways that are similar and correspond to the major biological themes. The WordCloud application was used to summarize these clusters based on word frequency within the pathway names.
Molecular docking and simulation
CB-Dock23, an online server for protein–ligand blind docking, was employed to assess the binding affinity and compatibility with the target protein [19]. CB-Dock2 integrates the pocket information based on a homologous template-based blinding docking procedure and the curvature-based cavity detection followed by AutoDock Vina4 for template-independent blind docking, which displays high accuracy in binding site identification and binding pose prediction. The full-length 3D structure of the mouse Hsp90aa protein was modeled with ColabFold5, which uses AlphaFold for structure prediction and combines a fast homology search against the crystal structure download from the Protein Data Bank (PDB ID: 5NJX) with default parameters. Then we submitted the protein and ligand, CB2-Dock2 will detect the cavity based on the ligand topology similarity and then return the refined docking poses. The visualization of the structure was implemented in PyMOL.
Small interfering RNA
The HSP90 siRNA (5′-CCCACTGCTTCTTTGTATA-3′) was synthesized by RiboBio (Guangzhou, China). The transfection was conducted using Lipofectamine RNAiMAX (Invitrogen), a reagent that facilitates the delivery of RNA interference (RNAi) molecules into cells. The expression levels of HSP90 were quantified using qPCR and western blotting.
Statistical analysis
Each experiment was conducted in triplicate. The data were represented as the mean ± SD, and statistical significance was examined using GraphPad Prism9.0 (San Diego, CA, United States). For multiple groups, statistical significance was analyzed using a one-way analysis of variance and Dunnett’s multiple comparisons test. p-values < 0.05 were considered statistically significant.
Results
Effect of lithospermic acid on the cell viability and ROS production with and without LPS stimulation
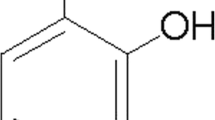
In order to ascertain the effect of lithospermic acid (Fig. 1A) on cell viability, a CCK-8 assay was performed 24 h after administration of different doses of lithospermic acid ranging from 3.125 to 100 µM. The CCK-8 assay revealed that lithospermic acid treatment did not exhibit cytotoxicity, compared to the untreated group (Fig. 1B). Therefore, lithospermic acid (25, 50, and 100 µM) was used in the experiments that followed.
Effect of lithospermic acid on the cell viability and ROS production with and without LPS stimulation. A The two-dimensional (2D) and three-dimensional (3D) chemical structures of lithospermic acid. B BV2 cells were treated with varying concentrations of lithospermic acid for a period of 24 h. C Prior to incubation with LPS (1 µg/ml) for 24 h, the cells were pre-treated with lithospermic acid at concentrations of 25, 50, and 100 µM for 1 h. Cell viability was determined by the CCK-8 assay. *p < 0.05, ***p < 0.001, ****p < 0.0001; ns, not significant. D Prior to incubation with LPS (1 µg/ml) for 24 h, the cells were pre-treated with lithospermic acid (100 µM) for 1 h. Cells were fixed with paraformaldehyde followed by staining with crystal violet solution. The morphological changes in different groups were observed and captured with microscopy. Scale bars, 50 µM. E The cells were treated as described in D and loaded with DCFH-DA, and immunofluorescence images were captured by fluorescence microscopy showing the ROS generation within the cells. Scale bars, 50 µM. F Mean fluorescence intensity of DCFH was quantified by ImageJ software. ****p < 0.0001; ns, not significant. LA, lithospermic acid
To mimic neuroinflammation, we proceeded to utilize LPS to prime BV2 cells as an experimental model. The viability of BV2 cells was significantly reduced by LPS (1 µg/ml) stimulation as indicated by the CCK-8 assay. Notably, lithospermic acid pre-treatment dose-dependently increased the cell viability against LPS-primed BV2 cells (Fig. 1C). In the normal control group, BV2 cells exhibited a distinctive morphological feature, characterized by small cytosomes that were primarily round or round-like in shape, with a notable absence of protrusions. Following stimulation with LPS, BV2 cells displayed significantly more prominent cytosomes, with an increased number of protrusions. Additionally, small spines were observed, exhibiting a typical “amoeboid” appearance, which is characteristic of activated microglia. Upon lithospermic acid treatment, LPS-stimulated BV2 cells had fewer branches that were shorter and or appeared to be resorbed into the cytosomes (Fig. 1D). Stimulation of microglial cells by LPS leads to an over-activation that can increase the generation of ROS, resulting in the induction of oxidative stress [20]. To evaluate whether the anti-neurotoxic effects of lithospermic acid are mediated by a reduction in ROS generation, the DCFH-DA probe was employed to quantify ROS production at the cellular level. The intensity of the DCFH fluorescence was markedly elevated following treatment with LPS. Nevertheless, pre-treatment with lithospermic acid resulted in a marked decrease in ROS fluorescence intensity in BV2 cells (Fig. 1E, F). Taken together, these results suggested that lithospermic acid pre-treatment exerts an inhibitory impact on LPS-related cytotoxicity in BV2 cells, which may be attributed to the reduction in ROS generation in LPS-primed cells.
Lithospermic acid inhibits the expression and secretion of pro-inflammatory cytokines by inhibiting the NF-κB signalling pathway in LPS-primed cells
The NF-κB/p65 signalling pathway plays a pivotal role in regulating the cellular immune response and in controlling the expression of a multitude of genes related to inflammation in activated microglia. It can be reasonably deduced that the inhibition of NF-κB activation may prove an effective strategy for the reduction of microglial-mediated neuroinflammation [21, 22]. In order to determine whether pre-treatment with lithospermic acid could inhibit NF-κB activation induced by LPS, we initially analyzed the localization of NF-κB p65 using immunofluorescence assays. Immunofluorescence images (Fig. 2A) revealed that NF-κB p65 had a main distribution in the cytosol in the control group. LPS primed can trigger most of the NF-κB p65 transfer from the cytosol into the nucleus. NF-κB p65 translocation was completely reversed after pretreatment with lithospermic acid (Fig. 2A). Furthermore, immunoblot analysis indicated that, upon LPS stimulation, NF-κB p65 levels were reduced in the cytosol, whereas they were increased in the nucleus (Fig. 2B–D). In contrast, NF-κB p65 translocation into the nucleus was markedly decreased after the pretreatment with lithospermic acid, which did not show any difference compared to the control group (Fig. 2B–D). It can be posited that the anti-inflammatory effect of lithospermic acid in LPS-primed cells is attributable to the inhibition of NF-κB/p65 pathway activation.
Lithospermic acid inhibits the expression and secretion of pro-inflammatory cytokines by inhibiting NF-KB signalling pathway in LPS-primed cells. The cells were treated as illustrated in Fig. 1D. A The immunofluorescence images showing the distribution of NF-κB p65 (red) in cells. Scale bars, 50 µM. B NF-κB p65 translocation was determined by immunoblotting assay. The loading control for the cytosol and nuclear lysates were β-Actin and Histone H3, respectively. The gray value of NF-κB p65 bands in the cytoplasm (C) or nucleus (D) was analyzed by AlphaEaseFC 4.0 software. **p < 0.01; ns, not significant. The mRNA expression levels of IL-6 (E), IL-1β (F), and TNF-α (G) were determined by real-time PCR. The culture supernatants cytokines IL-6 (H), IL-1β (I), and TNF-α (J) were measured by ELISA kits. **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to LPS group. LA, lithospermic acid
NF-κB pathway is crucial for the induction of the inflammatory cytokine genes transcription in activated BV2 microglia [11]. To further investigate the role of lithospermic acid in the LPS-induced inflammatory response, the expression and secretion of several key pro-inflammatory cytokines were quantified using real-time PCR and ELISA, respectively. Initially, real-time PCR was performed to determine whether the pre-treatment with lithospermic acid had the effect of suppressing the expression of these cytokines at the transcriptional level. The mRNA expression levels of IL-6, IL-1β, and TNF-α exhibited a notable elevation following LPS stimulation. In contrast, pre-treatment with lithospermic acid dose-dependently suppressed LPS-primed IL-6 (Fig. 2E), IL-1β (Fig. 2F), and TNF-α (Fig. 2G) mRNA transcription. Subsequently, ELISA was employed to determine the effects of lithospermic acid on LPS-induced inflammatory cytokines production. The results demonstrate that the production of IL-6, IL-1β, and TNF-α was markedly elevated following 24 h of LPS treatment. Furthermore, pre-treatment with lithospermic acid significantly reduced the production of inflammatory cytokines in LPS-primed BV2 cells in a dose-dependent manner. (Fig. 2H–J). These results indicated that lithospermic acid inhibits the expression and secretion of pro-inflammatory cytokines by inhibiting NF-KB signalling pathway in LPS-primed cells.
Lithospermic acid reduced COX2-mediated PGE2 generation in LPS-primed cells
COX2 expression is responsible for the activation of microglia and the resultant neuronal degeneration observed in neurodegenerative diseases [8]. Real-time PCR, immunofluorescence, and immunoblot were used to determine the inhibitory effects of lithospermic acid on COX2 mRNA and protein expression. Following a 24-h treatment with LPS, a significant upregulation was observed in both the mRNA and protein levels of COX2. Nevertheless, it was observed that the pretreated lithospermic acid demonstrated a dose-dependent inhibition of COX-2 mRNA and protein expression (Fig. 3A–D).
Effect of lithospermic acid on COX2 expression and PGE2 generation in LPS-primed BV2 cells. The cells were treated as illustrated in Fig. 1D. A Real-time PCR was used to quantify COX2 mRNA expression level. B Immunofluorescence images showing the subcellular expression of COX2 (red). Scale bars, 20 µM. C Cell lysates were analyzed by immunoblot to determine COX2 protein expression level. D Analysis of the gray value of COX2 bands was executed utilizing the AlphaEaseFC 4.0 software, where the LPS group values were standardized to 1.0, and values from other groups were compared to the LPS group. E PGE2 levels were determined by ELISA kit. ***p < 0.001, ****p < 0.0001. LA, lithospermic acid
COX-2 is a crucial rate-limiting enzyme in prostaglandin synthesis, which facilitates the synthesis and release of PGE2 in response to inflammatory stimuli. Accordingly, an evaluation was conducted to ascertain the inhibitory impact of lithospermic acid on the concentration of PGE2 in the supernatant. After incubating for 24 h, the culture medium was harvested, and the concentration of PGE2 was determined using an ELISA. PGE2 levels in the culture medium increased from baseline after 24 h following LPS stimulation. Figure 3E illustrates that pre-treatment with lithospermic acid markedly reduced the generation of PGE2 in BV2 cells primed with LPS. Based on these findings, lithospermic acid inhibits the expression of COX2 and the synthesis of PGE2 in LPS-primed cells, suggesting an anti-inflammatory effect.
Lithospermic acid reduced iNOS-mediated NO generation in LPS-primed cells
Activation of microglia during neuroinflammation results in an elevated secretion of nitric oxide, which is facilitated by the overexpression of iNOS. Next, we examined the effects of lithospermic acid on iNOS expression induced by LPS. The expression of iNOS was markedly enhanced in LPS-primed BV2 cells, as demonstrated by real-time PCR, immunofluorescence, and immunoblot analyses (Fig. 4A–D). The treatment of lithospermic acid effectively reduced the expression levels of LPS-induced iNOS mRNA (Fig. 4A) and protein (Fig. 4B, C) in a dose-dependent manner. Nitrite secretion into the culture supernatant was used to represent NO generation. Culture supernatants were measured using the Griess reagent to determine nitrite levels. It is noteworthy that the levels of nitrite were increased in BV2 cells when stimulated by LPS. The treatment with lithospermic acid resulted in a significant reduction in nitrite production (Fig. 4E). These results suggested that lithospermic acid has an inhibitory effect on the induction of iNOS and the generation of NO in LPS-primed BV2 cells.
Effect of lithospermic acid on iNOS expression and NO generation in LPS-primed BV2 cells. The cells were treated as illustrated in Fig. 1D. A Real-time PCR was used to quantify the iNOS mRNA expression level. B Immunofluorescence images showing the subcellular expression of iNOS (red). Scale bars, 20 µM. C Cell lysates were analyzed by immunoblot to determine iNOS protein expression level. D Analysis of the gray value of iNOS bands was executed utilizing the AlphaEaseFC 4.0 software, where the LPS group values were standardized to 1.0, and values from other groups were compared to the LPS group. E Griess reagent was used to detect NO generation. ***p < 0.001, ****p < 0.0001. LA, lithospermic acid
Effect of lithospermic acid on LPS-primed cell migration
The study demonstrated that the inflammatory responses are closely linked to the active migration of microglial cells [23]. Thus, we investigated whether lithospermic acid could inhibit LPS-primed cell migration. The results of the wound healing and transwell assays indicated that following stimulation with LPS, there was a significant promotion of the migration of BV2 cells. Lithospermic acid pre-treatment can significantly inhibit the migration of LPS-primed BV2 cells (Fig. 5). The results indicated that lithospermic acid has the potential to inhibit LPS-primed BV2 cell migration, which could contribute to the reduction of neuroinflammation.
Lithospermic acid inhibited the migration of LPS-primed BV2 cells. The cells were treated as illustrated in Fig. 1D. A Wound healing migration assay was performed, and bright-field images were acquired before and 24 h after treatment. B Quantitative analysis of BV2 cells migration rate was measured using ImageJ software. C Cell migration was assessed by transwell assays. Crystal violet staining was used to visualize migrated cells. Scale bars, 50 µM. D The invaded cell count was determined through the utilization of the ImageJ software. ****p < 0.0001. LA, lithospermic acid
Computational analysis of lithospermic acid targets and activity
To elucidate the molecular mechanisms by which lithospermic acid mitigates LPS-induced cytotoxicity in BV2 cells, we employed the SwissTargetPrediction tool for in silico target identification. This computational approach yielded a list of 100 putative gene targets for lithospermic acid (Table S1). Analysis of these genes revealed a diverse functional categorization: a predominant 33% of these identified genes were classified as kinases, representing the largest category, then followed by significant proportions of genes belonging to the “Family A G protein-coupled receptor” (21%), “Enzyme” (8%), and “Electrochemical transporter” (5%). The remaining gene targets were distributed across a spectrum of functional classes, including “Eraser,” “Family B G protein-coupled receptor,” “Hydrolase,” “Primary active transporters,” and “Ligand-gated ion channel” (Fig. 6A). Intriguingly, BV2 cells subjected to LPS-induced activation, characterized by aberrant cytosome morphology, demonstrated a reversal of this phenotype upon lithospermic acid treatment. This observation raised the hypothesis that genes differentially expressed in LPS-primed BV2 cells might be implicated in the observed cytotoxicity and could thus serve as potential targets for lithospermic acid. Integrating data from the SwissTargetPrediction with differentially expressed genes in the LPS model, we identified 42 overlapping genes as potential targets (Fig. 6B). Subsequently, we utilized the STRING database to construct a PPI network based on these 42 genes. The resulting network comprised 42 nodes and 85 edges. A detailed analysis of the network topology revealed several hub genes, including Stat3, Mmp9, Ptgs2, Hsp90aa1, and FYN, each exhibiting a connection degree exceeding 10 (Fig. 6C). Notably, within this network, 24 out of the 42 genes, accounting for 57.1%, were upregulated (Fig. 6D). Additionally, a significant proportion (80%) of the identified hub genes were also found to be upregulated.
Computational prediction of lithospermic acid targets and associated KEGG pathway enrichment and network analysis. A A pie chart, derived from the SwissTargetPrediction database, illustrates the categorization of target genes associated with lithospermic acid. B A Venn diagram shows the overlap between potential target genes of lithospermic acid and genes differentially expressed following LPS treatment in BV2 cells, highlighting the genes potentially modulated by lithospermic acid in an LPS-induced cellular context. C The PPI network map visualizes the interactions among the overlapping genes identified in B. Each node (circle) represents a protein, with the node size correlating to the number of interactions. The network’s connectivity is depicted through lines between nodes. Gene regulation is color-coded: downregulated genes in LPS-primed BV2 cells are shown in green, while upregulated genes are in red. D Heatmap showing the normalized expression values of the overlapping genes identified in B in both BV2 and LPS-primed BV2 cells. The color gradient, representing the z-score, indicates the level of gene expression. E Bar plot showing the significant enriched KEGG pathways using DAVID database with p-value ≤ 0.05. F An enrichmentMap clusters the potential target genes using the KEGG pathway database. Node size represents the degree of connectivity, with larger nodes (marked in red) indicating a higher number of interactions in proteins and smaller nodes (marked in green) indicating fewer interactions
To gain biological insights from these target genes, we utilized the DAVID database to conduct pathway enrichment analysis. A total of 29 overrepresented pathways were identified under the threshold of p-value ≤ 0.05 (Table S2). Among these collected pathways, the innate immune system was the most significant enriched pathway, characterized by a p-value = 4.69 × 10–4. Other enriched signalling pathways included “G alpha signalling events,” “GPCR downstream signalling,” “signalling by SCF-KIT,” “cytokine signalling in the immune system,” signal transduction, and “signalling by GPCR.” These findings suggest potential mechanisms by which lithospermic acid might have effects on these signalling-related genes. Besides, another notable finding was related to collagen-related pathways, including “collagen degradation,” “collagen formation,” and “assembly of collagen fibrils and other multimeric structures.” Pathways involved in the interaction between ligand and receptor were also observed, including “Class A/1 (Rhodopsin-like receptors),” “GPCR ligand binding,” and “Adenosine P1 receptors” (Fig. 6E). We next performed pathway-based enrichment analysis to investigate the functional connections within these pathways in the g: Profiler platform. After enrichment mapping and auto-annotation, the identified pathways were systematically clustered into 2 modules, including “immune systems,” and “signalling” (Fig. 6F). The topology of this pathway network showed that the “signalling transduction” was the largest pathway module, followed by the “immune system” module. The “signalling transduction” module consists of 7 pathways, including all the GPCR ligand-related pathways and several receptor-related pathways, such as the “Class A/1 (Rhodopsin-like receptors)” and “Adenosine P1 receptors.” Notably, strong connections between the “immune system” module and “signalling” module were observed, highlighting the intrinsic interrelations and shared functionalities within these pathways.
Lithospermic acid inhibits the activity and expression of HSP90
Based on these findings, we postulate that lithospermic acid may interact with these hub genes, potentially inhibiting their activity. To further explore the potential binding affinity of lithospermic acid to key hub genes, we conducted molecular docking to determine the likelihood of these candidate targets forming complexes with lithospermic acid (Fig. 7A). Notably, Hsp90aa, a molecular chaperon that aids protein folding, emerged as a gene of interest due to its upregulation in response to LPS treatment and its high degree of connectivity. Molecular docking analysis revealed the interaction between lithospermic acid and Hsp90aa. Specifically, residues including glutamine at 133, tryptophan at 314, and leucine at 352 were found to form hydrogen bonds with lithospermic acid (Fig. 7B). This interaction suggests a potential mechanism by which lithospermic acid could counteract the cytotoxic phenotype induced in LPS-primed BV2 cells, highlighting its promising role in modulating neuroimmunology responses. It remains unclear whether lithospermic acid interacts with HSP90aa, which has the potential to affect the activity of HSP90. As previously referenced, the inhibition of HSP90 results in an elevated expression of heat shock protein 70 (HSP70) [24], which may be regarded as a positive indicator of HSP90 inhibitor action. We next explored whether lithospermic acid could influence the expression of HSP70. The results of the immunoblot analysis indicated that treatment with lithospermic acid resulted in a dose-dependent increase in the expression of HSP70, which provides indirect evidence that lithospermic acid can inhibit the activity of HSP90 (Fig. 7C, D). In addition to its activity, we further tested whether lithospermic acid would affect the expression level of the HSP90 protein. The effects of varying concentrations of lithospermic acid were investigated in the presence or absence of LPS stimulation Immunoblot analysis showed that lithospermic acid treatment dose-dependently decreased the expression of HSP90 (Fig. 7E–H). This suggests that HSP90 may represent a pivotal target for lithospermic acid, which exerts its anti-inflammatory effects.
Lithospermic acid inhibits the activity and expression of HSP90. A The three-dimensional structure of the lithospermic acid-HSP90 complex is presented, with the full-length HSP90 structure modeled using AlphaFold2. The interaction between lithospermic acid and HSP90 is elucidated through CB2-dock analysis. B A two-dimensional interaction plot details key residue interactions within the complex, with hydrogen bonds illustrated as green lines, offering a detailed view of the molecular interactions at play. Cells were treated with varying concentrations of lithospermic acid, in the presence or absence of LPS stimulation. Cell lysates were analyzed by immunoblot to determine HSP70 (C, D) and HSP90 protein expression levels (E, F, G, H). Analysis of the gray value of HSP90 bands was executed utilizing the AlphaEaseFC 4.0 software. **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, not significant. LA, lithospermic acid
Knockdown of HSP90 attenuates inflammatory response after LPS stimulation in BV2 cells
To further confirm the role of HSP90 in mediating LPS-induced inflammation in BV2 cell, siRNA was recruited to knock down the expression of HSP90. qPCR and immunoblot showed that HSP90 expression was markedly decreased by HSP90-specific siRNA compared to the negative control group (Fig. 8A–C). Immunofluorescence microscopy and immunoblot showed that knockdown of HSP90 abrogated LPS-induced increase in COX2 and iNOS (Fig. 8D–F). In line with the lithospermic acid treatment, HSP90 knockdown significantly reduced the production of inflammatory cytokines, including IL-6 (Fig. 8G), IL-1β (Fig. 8H), and TNF-α (Fig. 8I) in LPS-primed BV2 cells. The aforementioned findings collectively indicate that lithospermic acid indeed regulates the activity and expression of HSP90, thereby attenuating the LPS-induced inflammatory response.
Knockdown of HSP90 attenuates inflammatory response after LPS stimulation in BV2 cells. Cells were knocked down by siRNA targeting the HSP90 gene. Negative control siRNA (NC group) was recruited as a knockdown control. Seventy-two hours after knockdown, cells were lysed and determined by qPCR (A) and western blotting (B, C). D Representative immunofluorescence images showing COX2 and iNOS subcellular distribution. Scale bars, 20 µM. E Cell lysates were analyzed by immunoblot to determine COX2 and iNOS protein expression level. F Analysis of the gray value of COX2 and iNOS bands was executed utilizing the AlphaEaseFC 4.0 software. ****p < 0.0001. The mRNA expression levels of IL-6 (G), IL-1β (H), and TNF-α (I) were determined by real-time PCR. ***p < 0.001; ****p < 0.0001
Discussion
It is increasingly demonstrated that certain traditional Chinese herbs and their active components possess considerable significant potential for treating various diseases, particularly immune-related disorders. The well-known medicinal plant Radix Salvia miltiorrhiza, also known as “Danshen” in Chinese, is the root of Salvia miltiorrhiza Bunge (Labiatae). It has been used in traditional Chinese folk medicine for the treatment of coronary heart disease, hypertension, renal disease, and myocardial infarction [25]. Lithospermic acid (LA) is a plant-derived polycyclic phenolic carboxylic acid isolated from Salvia miltiorrhiza. A growing number of researchers are concentrating their efforts on investigating the impact of lithospermic acid on inflammatory processes and associated pathologies. These therapeutic and beneficial effects include inhibition of HIV-1 replication [26], anti-oxidative and hepatoprotective activity on carbon tetrachloride (CCl4)-induced acute hepatic oxidative damage in vitro and in vivo [27], exhibiting anti-inflammatory effect in a model of gouty arthritis [28], improving cardiac function, and attenuating myocardial inflammation in the myocardial ischemia and reperfusion injury [29]. Furthermore, lithospermic acid has been demonstrated to mitigate neurotoxicity caused by 1-methyl-4-phenylpyridine through the inhibition of oxidative stress and neuroinflammation, indicating the potential of lithospermic acid as a novel therapeutic intervention for Parkinson’s disease [29]. Therefore, continuous investigations into the anti-inflammatory properties of lithospermic acid may advance the treatment of more clinical diseases.
In the CNS, microglia play a pivotal role in neuroprotection, which represents the first line of defense in the brain and initiates an immune response against injuries and stimuli [3, 30]. However, the aberrant activation of microglia ultimately leads to the death of neurons and an inflammatory response [31,32,33]. These neurotoxic and inflammatory factors contribute to the etiology of neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and other conditions associated with trauma and cerebral ischemia [3]. Hence, targeting the microglial activation process may prove an efficacious therapeutic strategy for numerous neurodegenerative disorders.
Murine cell line BV2 has been extensively characterized and used as a beneficial model for microglia due to their morphological, phenotypic, and functional similarities to primary microglia [34, 35]. The evidence accumulated thus far has firmly established that LPS has the ability to activate BV2 cells, leading to the production of various pro-inflammatory mediators and cytokines. For this reason, LPS-primed BV2 microglial cells have often been used as an in vitro model of neuroinflammation [36]. Our study found that the translocation of NF-κB p65 induced by LPS was completely reversed after pre-treatment with lithospermic acid. Lithospermic acid markedly decreased the production of pro-inflammatory mediators (ROS, NO, and PEG2) induced by LPS, and it also curbed the release of inflammatory cytokines (IL-6, IL-1β, and TNF-α) in BV2 microglial cells. The anti-inflammatory properties of lithospermic acid may be attributed to the inhibition of the expression of iNOS and COX2. Lithospermic acid treatment can significantly inhibit the migration of LPS-primed BV2 cells, which could contribute to the reduction of neuroinflammation. Further analysis has revealed that lithospermic acid interacts with HSP90, thereby inhibiting its activity and expression in a dose-dependent manner. In line with the lithospermic acid treatment, HSP90 knockdown significantly reduced the production of inflammatory cytokines. Overall, these results suggest that lithospermic acid targeting HSP90 attenuates LPS-induced inflammatory response via the NF-κB signalling pathway in BV2 microglial cells.
The maintenance of normal cell functions, such as immune response, neurotransmission, and regulation of blood pressure, is highly dependent on physiological levels of NO. Under conditions of inflammation following CNS injury, microglia that have been activated are capable of promoting the expression of iNOS, which catalyzes the production of NO by L-arginine. However, excessive NO production can lead to oxidative damage and ultimately result in cell death [37]. Likewise, inflammatory stimuli highly induce COX2, a crucial enzyme necessary for converting arachidonic acid into prostaglandin E2, which is the primary origin of PGE2 in numerous disorders such as cancer and inflammation [38]. COX2 is an inducible enzyme with low expression and activity in cells, but its expression significantly increases in response to stimulation with inflammatory factors. Multiple research reports have indicated that over-stimulation of COX2 corresponds with cytotoxicity in neurological disorders. This is further supported by evidence showing that the suppression of COX2 expression and/or activity mitigates the impact of brain damage following ischemia and decelerates the advancement of conditions such as AD and PD [39]. Prostaglandins are an important class of inflammatory mediators that mediate the initiation and spread of inflammation, with PGE2 having the more potent pro-inflammatory effect and being the most studied in diseases associated with cognitive dysfunction [40]. It was observed that LPS-primed BV2 microglial cells expressed more iNOS and COX2, resulting in elevated levels of NO and PGE2 in neurotoxicity. We demonstrated that lithospermic acid significantly reduced LPS-induced NO and PGE2 production in BV2 microglia cells without causing cytotoxicity. In addition, we were able to show that the lithospermic acid-mediated inhibition of PGE2 and NO production was caused by the suppression of both the mRNA and protein levels of COX2 (Fig. 3) and iNOS (Fig. 4) in LPS-primed BV2 cells. Prior research has demonstrated that HSP90 interacts with iNOS and COX2, with this interaction capable of regulating activity and subsequent NO and PGE2 production [41, 42]. To further investigate the role of HSP90 in mediating LPS-induced inflammation in BV2 cell, siRNA was recruited to knock down the expression of HSP90. It is interesting to note that the knockdown of HSP90 resulted in the abrogation of the LPS-induced increase in COX2 and iNOS, which aligns with the effects observed following lithospermic acid treatment (Fig. 8). Overall, suppression of iNOS and COX2 expression through the inhibition of HSP90 protein by lithospermic acid may have beneficial effects in reducing neuroinflammation.
Elevated levels of proinflammatory cytokines produced by activated microglia, including IL-6, IL-1β, and TNF-α, further modulate inflammation [43]. Therefore, inhibiting the production of pro-inflammatory cytokines is vital to preventing neuroinflammation. In this research, the LPS stimulation of BV2 microglia cells has significantly increased the production of these pro-inflammatory cytokines, which is consistent with earlier findings [44]. Our results demonstrated that the synthesis of the pro-inflammatory cytokines IL-6, IL-1β, and TNF-α was dose-dependently and markedly suppressed by lithospermic acid (Fig. 2E–J). Additionally, lithospermic acid reduced the mRNA expression levels of IL-6, IL-1β, and TNF-α, indicating that lithospermic acid suppressed the production of these cytokines by downregulating their gene expression in BV2 microglial cells stimulated by LPS as demonstrated in Fig. 2 E–G. The results indicated that lithospermic acid functions as an anti-inflammatory agent by blocking the release of inflammatory cytokines, which reduces the development of neuroinflammatory disorders that result from activated microglia. Additionally, our findings revealed that knockdown of HSP90 resulted in a reduction in the production of IL-6, IL-1β, and TNF-α. Nevertheless, this decline was not as significant as that observed following the administration of lithospermic acid. This discrepancy may be attributed to the fact that lithospermic acid is capable of not only inhibiting the expression of HSP90 but also of impeding the activity of HSP90. Further experimentation is required to substantiate this hypothesis.
Aside from inflammatory stimuli, oxidative stress represents another endogenous factor leading to damaged neurons. Many previous studies have established a direct relationship between the inflammatory responses by LPS in microglia and the increased generation of ROS. ROS production can be detrimental to the CNS as it can damage protein function and contribute to neurodegenerative disorders. Additionally, blocking ROS generation has been linked to the inhibition of inflammatory responses, as reported in previous studies. Generating ROS accelerates the inflammatory response by activating the NF-кB pathway [20, 45]. Hence, suppressing the production of ROS is a vital strategy for reducing both oxidative stress and inflammatory responses. According to our data presented in Fig. 1, it has been demonstrated that lithospermic acid is capable of effectively preventing the production of excessive ROS caused by LPS. It is proposed that the effect of lithospermic acid on neuroinflammation is likely to be mediated by its antioxidant property in BV2 microglial cells. Further studies are required to determine the direct relationship between blocking ROS generation and anti-inflammatory action.
A plethora of evidence indicated that NF-κB controls the transcription of various genes that encode neurotoxic inflammatory mediators (iNOS and COX2) as well as cytokines (IL-6, IL-1β, and TNF-α) [46]. NF-кB is known to play a critical role in microglia-mediated inflammatory responses. In the resting state, the NF-кB p65 is mainly distributed in the cytosol and combined with the inhibitory protein IкBα. IкBα is phosphorylated and rapidly degraded upon microglial activation by stimuli such as LPS. This process triggers the separation of NF-κB from IκBα, which allows it to translocate into the nucleus and then cause certain inflammatory genes to be transcribed [47]. In our current investigation, LPS treatment significantly induced the translocation of the NF-κB p65 subunit into the nucleus, which is consistent with the findings of previous research [21]. The results demonstrated that lithospermic acid significantly reduced the nuclear translocation of NF-κB p65 in response to LPS in BV2 microglial cells (Fig. 2). Accordingly, our results indicate that lithospermic acid inhibits the activated NF-кB pathway in order to decrease iNOS, COX2, and inflammatory cytokines.
Interestingly, microglia will be recruited to the site of injury or infection by factors released by damaged cells. Thus, it is widely acknowledged that the activation of microglia is closely linked to cell migration, resulting in tissue damage and chronic inflammation [12]. NO produced in the lesion sites functions importantly as a signal, urging cell migration during inflammation [37]. Given our discovery that lithospermic acid dramatically inhibits NO generation (Fig. 4), we next investigated whether it could affect the LPS-induced cell migration. As expected, lithospermic acid dramatically reduced the migration of LPS-primed cells (Fig. 5), suggesting that it has the potential to abrogate LPS-primed cell migration and thereby ameliorate neuroinflammation.
However, it is important to acknowledge that our current study does not include detailed pharmacokinetic data or an extensive toxicity study for LA in vivo. While our preliminary computational ADMET profiling prediction by the OptADMET [48] database (https://cadd.nscc-tj.cn/deploy/optadmet/) provides an initial indication of LA’s potential druggable properties (Table S3), these in silico predictions cannot fully substitute for empirical pharmacokinetic and safety studies. In future research, it will be necessary to conduct thorough in vivo pharmacokinetic assessments, including bioavailability, half-life, metabolic stability tests, and comprehensive toxicity evaluations. Such investigations are essential steps before considering clinical translation and ensuring the efficacy and safety in LA in the therapeutic setting.
Conclusion
Our findings in this research are summarized in a schematic diagram (Fig. 9). The diagram illustrates the signalling pathways and mechanism through which lithospermic acid exerts its anti-inflammatory and antimigratory effects. In this study, we present evidence that lithospermic acid mitigates the activation of microglia and the subsequent pro-inflammatory reactions induced by LPS in BV2 microglial cells while maintaining cell viability. Lithospermic acid demonstrated the ability to suppress NF-κB activity and ROS accumulation, resulting in a reduction in the production of pro-inflammatory mediators and cytokines, as well as a decrease in the expression of their regulatory genes. Molecular docking and experimental verification have demonstrated that lithospermic acid inhibits the activity and expression of HSP90. Small interfering RNA knockdown of HSP90 expression, which abrogated LPS-induced inflammation. These findings suggest that the lithospermic acid targeting HSP90 attenuates LPS-induced inflammatory response via NF-κB signalling pathway. These findings indicate that lithospermic acid may hold promise as a therapeutic intervention for various neurodegenerative disorders characterized by neuroinflammation and microglial activation. Nevertheless, further in vivo investigations employing animal models are imperative to substantiate the effectiveness of lithospermic acid.
The schematic illustrates the possibility of lithospermic acid acting as a potential inhibitor of inflammatory responses in LPS-primed BV2 cells. LPS, lipopolysaccharide; TLR4, toll-like receptor 4; NF-κB, nuclear factor-kappa B; HSP90, heat shock protein 90; COX2, cyclooxygenase-2; PGE2, prostaglandin E2; iNOS, inducible nitric oxide synthase; NO, nitric oxide; IL-6, interleukin-6; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α
Data availability
No datasets were generated or analysed during the current study.
References
Wang Y, Gao H, Zhang W, Zhang W, Fang L. Thymoquinone inhibits lipopolysaccharide-induced inflammatory mediators in BV2 microglial cells. Int Immunopharmacol. 2015;26(1):169–73. https://doi.org/10.1016/j.intimp.2015.03.013.
Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17(3):157–72. https://doi.org/10.1038/s41582-020-00435-y.
Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154(2):204–19. https://doi.org/10.1111/imm.12922.
Fan Z, Aman Y, Ahmed I, Chetelat G, Landeau B, Ray Chaudhuri K et al. Influence of microglial activation on neuronal function in Alzheimer's and Parkinson's disease dementia. Alzheimers Dement. 2015;11(6):608–21 e7. https://doi.org/10.1016/j.jalz.2014.06.016
Zhang L, Gao J, Tang P, Chong L, Liu Y, Liu P, et al. Nuciferine inhibits LPS-induced inflammatory response in BV2 cells by activating PPAR-gamma. Int Immunopharmacol. 2018;63:9–13. https://doi.org/10.1016/j.intimp.2018.07.015.
Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–51. https://doi.org/10.1016/j.cyto.2008.01.006.
Smith AG, Kliebe VM, Mishra S, McCall RP, Irvine MM, Blagg BSJ, et al. Anti-inflammatory activities of novel heat shock protein 90 isoform selective inhibitors in BV-2 microglial cells. Front Mol Biosci. 2024;11:1405339. https://doi.org/10.3389/fmolb.2024.1405339.
Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63(9):901–10. https://doi.org/10.1093/jnen/63.9.901.
Yuste JE, Tarragon E, Campuzano CM, Ros-Bernal F. Implications of glial nitric oxide in neurodegenerative diseases. Front Cell Neurosci. 2015;9:322. https://doi.org/10.3389/fncel.2015.00322.
Yang S, Chu S, Ai Q, Zhang Z, Gao Y, Lin M, et al. Anti-inflammatory effects of higenamine (Hig) on LPS-activated mouse microglia (BV2) through NF-kappaB and Nrf2/HO-1 signaling pathways. Int Immunopharmacol. 2020;85:106629. https://doi.org/10.1016/j.intimp.2020.106629.
Zhu J, Jiang L, Liu Y, Qian W, Liu J, Zhou J, et al. MAPK and NF-kappaB pathways are involved in bisphenol A-induced TNF-alpha and IL-6 production in BV2 microglial cells. Inflammation. 2015;38(2):637–48. https://doi.org/10.1007/s10753-014-9971-5.
Dou Y, Wu HJ, Li HQ, Qin S, Wang YE, Li J, et al. Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res. 2012;22(6):1022–33. https://doi.org/10.1038/cr.2012.10.
Su CY, Ming QL, Rahman K, Han T, Qin LP. Salvia miltiorrhiza: traditional medicinal uses, chemistry, and pharmacology. Chin J Nat Med. 2015;13(3):163–82. https://doi.org/10.1016/S1875-5364(15)30002-9.
Li CG, Yan L, Mai FY, Shi ZJ, Xu LH, Jing YY, et al. Baicalin inhibits NOD-like receptor family, pyrin containing domain 3 inflammasome activation in murine macrophages by augmenting protein kinase A signaling. Front Immunol. 2017;8:1409. https://doi.org/10.3389/fimmu.2017.01409.
Zeng QZ, Yang F, Li CG, Xu LH, He XH, Mai FY, et al. Paclitaxel enhances the innate immunity by promoting NLRP3 inflammasome activation in macrophages. Front Immunol. 2019;10:72. https://doi.org/10.3389/fimmu.2019.00072.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. https://doi.org/10.1186/s13059-014-0550-8.
Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):P3.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. https://doi.org/10.1101/gr.1239303.
Liu Y, Yang X, Gan J, Chen S, Xiao ZX, Cao Y. CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022;50(W1):W159–64. https://doi.org/10.1093/nar/gkac394.
Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev. 2016;2016:2795090. https://doi.org/10.1155/2016/2795090.
Bui BP, Oh Y, Lee H, Cho J. Inhibition of inflammatory mediators and cell migration by 1,2,3,4-tetrahydroquinoline derivatives in LPS-stimulated BV2 microglial cells via suppression of NF-kappaB and JNK pathway. Int Immunopharmacol. 2020;80:106231. https://doi.org/10.1016/j.intimp.2020.106231.
Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309(5742):1854–7. https://doi.org/10.1126/science.1112304.
Nam HY, Nam JH, Yoon G, Lee JY, Nam Y, Kang HJ, et al. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J Neuroinflammation. 2018;15(1):271. https://doi.org/10.1186/s12974-018-1308-0.
Aherne W, Maloney A, Prodromou C, Rowlands MG, Hardcastle A, Boxall K, et al. Assays for HSP90 and inhibitors. Methods Mol Med. 2003;85:149–61. https://doi.org/10.1385/1-59259-380-1:149.
Li Y, Tu Z, Chen F, Yang X, Deng R, Su F, et al. Anti-inflammatory effect of Danhong injection through inhibition of GSDMD-mediated pyroptosis. Phytomedicine. 2023;113:154743. https://doi.org/10.1016/j.phymed.2023.154743.
Abd-Elazem IS, Chen HS, Bates RB, Huang RC. Isolation of two highly potent and non-toxic inhibitors of human immunodeficiency virus type 1 (HIV-1) integrase from Salvia miltiorrhiza. Antiviral Res. 2002;55(1):91–106. https://doi.org/10.1016/s0166-3542(02)00011-6.
Chan KW, Ho WS. Anti-oxidative and hepatoprotective effects of lithospermic acid against carbon tetrachloride-induced liver oxidative damage in vitro and in vivo. Oncol Rep. 2015;34(2):673–80. https://doi.org/10.3892/or.2015.4068.
Liu X, Chen R, Shang Y, Jiao B, Huang C. Lithospermic acid as a novel xanthine oxidase inhibitor has anti-inflammatory and hypouricemic effects in rats. Chem Biol Interact. 2008;176(2–3):137–42. https://doi.org/10.1016/j.cbi.2008.07.003.
Zhang M, Wei L, Xie S, Xing Y, Shi W, Zeng X, et al. Activation of Nrf2 by lithospermic acid ameliorates myocardial ischemia and reperfusion injury by promoting phosphorylation of AMP-activated protein kinase alpha (AMPKalpha). Front Pharmacol. 2021;12:794982. https://doi.org/10.3389/fphar.2021.794982.
Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147(4):867–83. https://doi.org/10.1016/j.neuroscience.2007.02.055.
Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. https://doi.org/10.1038/nrn2038.
Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304(1):1–7. https://doi.org/10.1124/jpet.102.035048.
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–34. https://doi.org/10.1016/j.cell.2010.02.016.
Standoli S, Rapino C, Di Meo C, Rudowski A, Kampfer-Kolb N, Volk LM et al. Sphingosine kinases at the intersection of pro-inflammatory LPS and anti-inflammatory endocannabinoid signaling in BV2 mouse microglia cells. Int J Mol Sci. 2023;24(10). https://doi.org/10.3390/ijms24108508
Barber K, Mendonca P, Evans JA, Soliman KFA. Antioxidant and anti-inflammatory mechanisms of cardamonin through Nrf2 activation and NF-kB suppression in LPS-activated BV-2 microglial cells. Int J Mol Sci. 2023;24(13). https://doi.org/10.3390/ijms241310872
Wang-Sheng C, Jie A, Jian-Jun L, Lan H, Zeng-Bao X, Chang-Qing L. Piperine attenuates lipopolysaccharide (LPS)-induced inflammatory responses in BV2 microglia. Int Immunopharmacol. 2017;42:44–8. https://doi.org/10.1016/j.intimp.2016.11.001.
Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8(10):766–75. https://doi.org/10.1038/nrn2214.
Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12(12):1063–73.
Giovannini MG, Scali C, Prosperi C, Bellucci A, Pepeu G, Casamenti F. Experimental brain inflammation and neurodegeneration as model of Alzheimer’s disease: protective effects of selective COX-2 inhibitors. Int J Immunopathol Pharmacol. 2003;16(2 Suppl):31–40.
Cudaback E, Jorstad NL, Yang Y, Montine TJ, Keene CD. Therapeutic implications of the prostaglandin pathway in Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):565–72. https://doi.org/10.1016/j.bcp.2013.12.014.
Kobayashi T, Nakatani Y, Tanioka T, Tsujimoto M, Nakajo S, Nakaya K, et al. Regulation of cytosolic prostaglandin E synthase by phosphorylation. Biochem J. 2004;381(Pt 1):59–69. https://doi.org/10.1042/BJ20040118.
Wang T, Luo S, Qin H, Xia Y. Hsp90 inhibition renders iNOS aggregation and the clearance of iNOS aggregates by proteasomes requires SPSB2. Free Radic Biol Med. 2018;117:90–8. https://doi.org/10.1016/j.freeradbiomed.2018.01.021.
Giulian D, Li J, Li X, George J, Rutecki PA. The impact of microglia-derived cytokines upon gliosis in the CNS. Dev Neurosci. 1994;16(3–4):128–36. https://doi.org/10.1159/000112099.
Park JS, Woo MS, Kim DH, Hyun JW, Kim WK, Lee JC, et al. Anti-inflammatory mechanisms of isoflavone metabolites in lipopolysaccharide-stimulated microglial cells. J Pharmacol Exp Ther. 2007;320(3):1237–45. https://doi.org/10.1124/jpet.106.114322.
Slusarczyk J, Trojan E, Glombik K, Piotrowska A, Budziszewska B, Kubera M, et al. Anti-inflammatory properties of tianeptine on lipopolysaccharide-induced changes in microglial cells involve toll-like receptor-related pathways. J Neurochem. 2016;136(5):958–70. https://doi.org/10.1111/jnc.13452.
Chow YL, Lee KH, Vidyadaran S, Lajis NH, Akhtar MN, Israf DA, et al. Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-gamma/LPS-stimulated BV2 microglia via NF-kappaB signalling pathway. Int Immunopharmacol. 2012;12(4):657–65. https://doi.org/10.1016/j.intimp.2012.01.009.
Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. https://doi.org/10.1186/1476-4598-12-86.
Yi J, Shi S, Fu L, Yang Z, Nie P, Lu A, et al. OptADMET: a web-based tool for substructure modifications to improve ADMET properties of lead compounds. Nat Protoc. 2024;19(4):1105–21. https://doi.org/10.1038/s41596-023-00942-4.
Acknowledgements
The illustration in Fig. 9 was created with figdraw.com. (ID: IWSPO14400).
Funding
This work was supported by grants from the National Natural Science Foundation of China (no. 82305387; no.82404670), GuangDong Basic and Applied Basic Research Foundation (no. 2023A1515110466), Shenzhen Nanshan District Health System Science and Technology Major Project Outstanding Youth Fund (no. NSZD2024035), and Shenzhen Science and Technology R&D Fund Basic Research Project (no. JCYJ20230807115813028, no. JCYJ20220530150407015).
Author information
Authors and Affiliations
Contributions
JG: Conceptualization, Formal Analysis,Writing–original draft, Funding acquisition. C-GL:Visualization, Data curation, Formal analysis, Investigation, Funding acquisition. F-YM: Writing–original draft, Writing–review & editing, Validation. J-RL: Conceptualization, Data curation, Funding acquisition. Z-HC: Methodology, Validation. JL:Investigation, Project administration,Validation. M-CZ: Investigation,Validation. Y-LW: Conceptualization, Methodology, Investigation, Software, Validation. W-TY: Writing-original draft, Writing-review & editing, Methodology, Supervision, Project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, J., Li, Cg., Mai, Fy. et al. Lithospermic acid targeting heat shock protein 90 attenuates LPS-induced inflammatory response via NF-кB signalling pathway in BV2 microglial cells. Immunol Res 73, 54 (2025). https://doi.org/10.1007/s12026-025-09600-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12026-025-09600-1