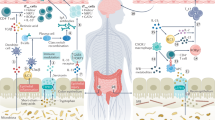
Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease that leads to demyelination and damage to the central nervous system. It is well known, the significance of the involvement and influence of the immune system in the development and course of MS. Nowadays, more and more studies are demonstrating that an important factor that affects the action of the immune system is the gut microbiota. Changes in the composition and interrelationships in the gut microbiota have a significant impact on the course of MS. Dysbiosis affects the disease course mainly by influencing the immune system directly but also by modifying the secreted metabolites and increasing mucosal permeability. The essential metabolites affecting the course of MS are short-chain fatty acids, which alter pro- and anti-inflammatory responses in the immune system but also increase the permeability of the intestinal wall and the blood–brain barrier. Dietary modification alone can have a significant impact on MS. Based on these interactions, new treatments for MS are being developed, including probiotics administration, supplementation of bacterial metabolites, fecal microbiota transplantation, and dietary changes. Further studies may serve to develop new drugs and therapeutic approaches for MS.


Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and can be accessed by DOI from references.
References
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. The Lancet. 2018;391(10130):1622–36. https://doi.org/10.1016/S0140-6736(18)30481-1.
Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention—an update. Semin Neurol. 2016;36(02):103–14. https://doi.org/10.1055/s-0036-1579693.
Howard J, Trevick S, Younger DS. Epidemiology of multiple sclerosis. Neurol Clin. 2016;34(4):919–39. https://doi.org/10.1016/j.ncl.2016.06.016.
Walton C, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler J. 2020;26(14):1816–21. https://doi.org/10.1177/1352458520970841.
Winquist RJ, Kwong A, Ramachandran R, Jain J. The complex etiology of multiple sclerosis. Biochem Pharmacol. 2007;74(9):1321–9. https://doi.org/10.1016/j.bcp.2007.04.026.
Sintzel MB, Rametta M, Reder AT. Vitamin D and multiple sclerosis: a comprehensive review. Neurol Ther. 2018;7(1):59–85. https://doi.org/10.1007/s40120-017-0086-4.
Soldan SS, Lieberman PM. Epstein–Barr virus and multiple sclerosis. Nat Rev Microbiol. 2023;21(1):51–64. https://doi.org/10.1038/s41579-022-00770-5.
Cappelletti C, et al. Quantitative proteomics reveals protein dysregulation during T cell activation in multiple sclerosis patients compared to healthy controls. Clin Proteomics. 2022;19(1):23. https://doi.org/10.1186/s12014-022-09361-1.
Chitnis T. The role of CD4 T cells in the pathogenesis of multiple sclerosis. Int Rev Neurobiol. 2007;79:43–72. https://doi.org/10.1016/S0074-7742(07)79003-7.
Mey GM, Mahajan KR, DeSilva TM. ‘Neurodegeneration in multiple sclerosis. WIREs Mech Dis. 2023;15:1. https://doi.org/10.1002/wsbm.1583.
Racke M. Immunopathogenesis of multiple sclerosis. Ann Indian Acad Neurol. 2009;12(4):215. https://doi.org/10.4103/0972-2327.58274.
Dhanapalaratnam R, Markoulli M, Krishnan AV. Disorders of vision in multiple sclerosis. Clin Exp Optom. 2022;105(1):3–12. https://doi.org/10.1080/08164622.2021.1947745.
Ghosh R, Roy D, Dubey S, Das S, Benito-León J. Movement disorders in multiple sclerosis: an update. Tremor Other Hyperkinetic Movements. 2022;12(1):14. https://doi.org/10.5334/tohm.671.
Lisak M, Špiljak B, Pašić H, Trkanjec Z. Cognitive aspects in multiple sclerosis. Psychiatr Danub. 2021;33(Suppl 13):177–82.
Potulska-Chromik A, et al. Original article clinical and neuroimaging correlation of movement disorders in multiple sclerosis: case series and review of the literature. Folia Neuropathol. 2014;1:92–100. https://doi.org/10.5114/fn.2014.41747.
Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31(3):252–6. https://doi.org/10.1016/j.jaut.2008.04.017.
Høglund RA. Multiple sclerosis and the role of immune cells. World J Exp Med. 2014;4(3):27. https://doi.org/10.5493/wjem.v4.i3.27.
Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KHG. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162(1):1–11. https://doi.org/10.1111/j.1365-2249.2010.04143.x.
Yuan X, et al. Functional and metabolic alterations of gut microbiota in children with new-onset type 1 diabetes. Nat Commun. 2022;13(1):6356. https://doi.org/10.1038/s41467-022-33656-4.
Xin X, Wang Q, Qing J, et al. Th17 cells in primary Sjögren's syndrome negatively correlate with increased Roseburia and Coprococcus. Front Immunol. 2022;13:974648. https://doi.org/10.3389/fimmu.2022.974648.
Freedman SN, Shahi SK, Mangalam AK. The “gut feeling”: breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics. 2018;15(1):109–25. https://doi.org/10.1007/s13311-017-0588-x.
Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–36. https://doi.org/10.1042/BCJ20160510.
Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. https://doi.org/10.1038/nature11053.
Senn V, Bassler D, Choudhury R, et al. Microbial colonization from the fetus to early childhood-a comprehensive review [published correction appears in Front Cell Infect Microbiol. 2021 Jun 30;11:715671]. Front Cell Infect Microbiol. 2020;10:573735. https://doi.org/10.3389/fcimb.2020.573735.
Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76(3):473–93. https://doi.org/10.1007/s00018-018-2943-4.
Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. https://doi.org/10.1016/j.cell.2014.03.011.
Mörbe UM, et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021;14(4):793–802. https://doi.org/10.1038/s41385-021-00389-4.
Kasarello K, Cudnoch-Jedrzejewska A, Czarzasta K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front Microbiol. 2023;14:1118529. https://doi.org/10.3389/fmicb.2023.1118529.
Kim M, Kim CH. Regulation of humoral immunity by gut microbial products. Gut Microbes. 2017;8(4):392–9. https://doi.org/10.1080/19490976.2017.1299311.
Linares DM, Ross P, Stanton C. Beneficial microbes: the pharmacy in the gut. Bioengineered. 2016;7(1):11–20. https://doi.org/10.1080/21655979.2015.1126015.
Macpherson AJ, Uhr T. (2004) ‘Induction of protective iga by intestinal dendritic cells carrying commensal bacteria.’ Science. 1979;303(5664):1662–5. https://doi.org/10.1126/science.1091334.
Sutherland DB, Fagarasan S. IgA synthesis: a form of functional immune adaptation extending beyond gut. Curr Opin Immunol. 2012;24(3):261–8. https://doi.org/10.1016/j.coi.2012.03.005.
Rinninella E, et al. ‘What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases.’ Microorganisms. 2019;7(1):14. https://doi.org/10.3390/microorganisms7010014.
Cignarella F, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018;27(6):1222-1235.e6. https://doi.org/10.1016/j.cmet.2018.05.006.
DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. 2016;22(5):1137–50. https://doi.org/10.1097/MIB.0000000000000750.
Wexler HM. Bacteroides : the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20(4):593–621. https://doi.org/10.1128/CMR.00008-07.
Zhang Y-J, Li S, Gan R-Y, Zhou T, Xu D-P, Li H-B. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16(12):7493–519. https://doi.org/10.3390/ijms16047493.
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:0. https://doi.org/10.3402/mehd.v26.26191.
Vacaras V, et al. The role of multiple sclerosis therapies on the dynamic of human gut microbiota. J Neuroimmunol. 2023;378:578087. https://doi.org/10.1016/j.jneuroim.2023.578087.
Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-Induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4(8):1095–119. https://doi.org/10.3390/nu4081095.
Buscarinu MC, et al. Intestinal permeability in relapsing-remitting multiple sclerosis. Neurotherapeutics. 2018;15(1):68–74. https://doi.org/10.1007/s13311-017-0582-3.
Hrncir T. Gut microbiota dysbiosis: triggers, consequences, diagnostic and therapeutic options. Microorganisms. 2022;10(3):578. https://doi.org/10.3390/microorganisms10030578.
Yacyshyn B, Meddings J, Sadowski D, Bowen-Yacyshyn MB. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig Dis Sci. 1996;41(12):2493–8. https://doi.org/10.1007/BF02100148.
Coombes JL, Maloy KJ. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Semin Immunol. 2007;19(2):116–26. https://doi.org/10.1016/j.smim.2007.01.001.
Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc National Acad Sci. 2011;108(supplement_1):4615–22. https://doi.org/10.1073/pnas.1000082107.
Chen J, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6(1):28484. https://doi.org/10.1038/srep28484.
Naghavian R, Ghaedi K, Kiani-Esfahani A, Ganjalikhani-Hakemi M, Etemadifar M, Nasr-Esfahani MH. miR-141 and miR-200a, revelation of new possible players in modulation of Th17/Treg differentiation and pathogenesis of multiple sclerosis. PLoS ONE. 2015;10(5):e0124555. https://doi.org/10.1371/journal.pone.0124555.
Seddiki N, Brezar V, Ruffin N, Lévy Y, Swaminathan S. Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology. 2014;142(1):32–8. https://doi.org/10.1111/imm.12227.
Zhou H, Li J, Gao P, Wang Q, Zhang J. miR-155: a novel target in allergic asthma. Int J Mol Sci. 2016;17(10):1773. https://doi.org/10.3390/ijms17101773.
Ivanov II, et al. Induction of Intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. https://doi.org/10.1016/j.cell.2009.09.033.
Pröbstel A-K, et al. Gut microbiota–specific IgA + B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. 2020;5:53. https://doi.org/10.1126/sciimmunol.abc7191.
Labbé A, Ganopolsky JG, Martoni CJ, Prakash S, Jones ML. Bacterial bile metabolising gene abundance in Crohn’s, ulcerative colitis and type 2 diabetes metagenomes. PLoS ONE. 2014;9(12):e115175. https://doi.org/10.1371/journal.pone.0115175.
Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183(10):6251–61. https://doi.org/10.4049/jimmunol.0803978.
Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD—what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9(4):219–30. https://doi.org/10.1038/nrgastro.2012.14.
Braniste V, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263. https://doi.org/10.1126/scitranslmed.3009759.
Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. https://doi.org/10.1080/19490976.2015.1134082.
Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. https://doi.org/10.3389/fmicb.2016.00185.
Mariadason JM, Catto-Smith A, Gibson PR. Modulation of distal colonic epithelial barrier function by dietary fibre in normal rats. Gut. 1999;44(3):394–9. https://doi.org/10.1136/gut.44.3.394.
Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61(1):37–41. https://doi.org/10.1203/01.pdr.0000250014.92242.f3.
Richards JL, Yap YA, McLeod KH, Mackay CR, Mariño E. Dietary metabolites and the gut microbiota: an alternative approach to control inflammatory and autoimmune diseases. Clin Transl Immunol. 2016;5(5):e82. https://doi.org/10.1038/cti.2016.29.
Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS ONE. 2017;12(2):e0173032. https://doi.org/10.1371/journal.pone.0173032.
Tankou SK, et al. Investigation of probiotics in multiple sclerosis. Mult Scler J. 2018;24(1):58–63. https://doi.org/10.1177/1352458517737390.
Mendozzi L, et al. Changing lifestyle of persons with multiple sclerosis: development, feasibility and preliminary results of a novel high-impact collaborative intervention in leisure environments. Int J Phys Med Rehabil. 2018;06:02. https://doi.org/10.4172/2329-9096.1000461.
Barone M, et al. Influence of a high-impact multidimensional rehabilitation program on the gut microbiota of patients with multiple sclerosis. Int J Mol Sci. 2021;22(13):7173. https://doi.org/10.3390/ijms22137173.
Jenkins TP, et al. Experimental infection with the hookworm, Necator americanus, is associated with stable gut microbial diversity in human volunteers with relapsing multiple sclerosis. BMC Biol. 2021;19(1):74. https://doi.org/10.1186/s12915-021-01003-6.
Zangeneh Z, Abdi-Ali A, Khamooshian K, Alvandi A, Abiri R. Bacterial variation in the oral microbiota in multiple sclerosis patients. PLoS ONE. 2021;16(11):e0260384. https://doi.org/10.1371/journal.pone.0260384.
Tankou SK, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. 2018;83(6):1147–61. https://doi.org/10.1002/ana.25244.
Lavasani S, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE. 2010;5(2):e9009. https://doi.org/10.1371/journal.pone.0009009.
Salehipour Z, et al. Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed Pharmacother. 2017;95:1535–48. https://doi.org/10.1016/j.biopha.2017.08.117.
Mangalam A, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 2017;20(6):1269–77. https://doi.org/10.1016/j.celrep.2017.07.031.
Shahi SK, Freedman SN, Murra AC, et al. Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal model of multiple sclerosis. Front Immunol. 2019;10:462. https://doi.org/10.3389/fimmu.2019.00462.
Leray E, Moreau T, Fromont A, Edan G. Epidemiology of multiple sclerosis. Rev Neurol (Paris). 2016;172(1):3–13. https://doi.org/10.1016/j.neurol.2015.10.006.
Berer K, et al. Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. Sci Rep. 2018;8(1):10431. https://doi.org/10.1038/s41598-018-28839-3.
Esposito S, Bonavita S, Sparaco M, Gallo A, Tedeschi G. The role of diet in multiple sclerosis: a review. Nutr Neurosci. 2018;21(6):377–90. https://doi.org/10.1080/1028415X.2017.1303016.
Choi IY, et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016;15(10):2136–46. https://doi.org/10.1016/j.celrep.2016.05.009.
Bai M, et al. Intermittent caloric restriction with a modified fasting-mimicking diet ameliorates autoimmunity and promotes recovery in a mouse model of multiple sclerosis. J Nutr Biochem. 2021;87:108493. https://doi.org/10.1016/j.jnutbio.2020.108493.
Ang QY, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181(6):1263-1275.e16. https://doi.org/10.1016/j.cell.2020.04.027.
Hoffman K, Doyle WJ, Schumacher SM, Ochoa-Repáraz J, ‘Gut microbiome-modulated dietary strategies in EAE and multiple sclerosis’, Front Nutr 2023; 10 https://doi.org/10.3389/fnut.2023.1146748.
Albrechtsen MT, Langeskov-Christensen M, Jørgensen MLK, Dalgas U, Hansen M. Is diet associated with physical capacity and fatigue in persons with multiple sclerosis? –results from a pilot study. Mult Scler Relat Disord. 2020;40:101921. https://doi.org/10.1016/j.msard.2019.101921.
Hedström AK, Hillert J, Olsson T, Alfredsson L. Alcohol as a modifiable lifestyle factor affecting multiple sclerosis risk. JAMA Neurol. 2014;71(3):300. https://doi.org/10.1001/jamaneurol.2013.5858.
Caslin B, Maguire C, Karmakar A, Mohler K, Wylie D, Melamed E. Alcohol shifts gut microbial networks and ameliorates a murine model of neuroinflammation in a sex-specific pattern. Proc Natl Acad Sci. 2019;116(51):25808–15. https://doi.org/10.1073/pnas.1912359116.
González-Quintela A, Dominguez-Santalla MJ, Pérez LF, Vidal C, Lojo S, Barrio E. Influence of acute alcohol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 AND IL-12. Cytokine. 2000;12(9):1437–40. https://doi.org/10.1006/cyto.2000.0715.
Browne AS, Kelly CR. Fecal transplant in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46(4):825–37. https://doi.org/10.1016/j.gtc.2017.08.005.
Vendrik KEW et al. ‘Fecal microbiota transplantation in neurological disorders’, Front Cell Infect Microbiol 2020; 10 https://doi.org/10.3389/fcimb.2020.00098.
Rakotonirina A, Galperine T, Allémann E. Fecal microbiota transplantation: a review on current formulations in Clostridioides difficile infection and future outlooks. Expert Opin Biol Ther. 2022;22(7):929–44. https://doi.org/10.1080/14712598.2022.2095901.
Engen PA et al. ‘Single-arm, non-randomized, time series, single-subject study of fecal microbiota transplantation in multiple sclerosis’. Front Neurol 2020; 11 https://doi.org/10.3389/fneur.2020.00978.
Makkawi S, Camara-Lemarroy C, Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol - Neuroimmunol Neuroinflammation. 2018;5(4):e459. https://doi.org/10.1212/NXI.0000000000000459.
Garcia-Rodriguez V, Ali SI, Dupont AW. S2314 Fecal microbiota transplantation associated with disease stabilization in a patient with multiple sclerosis. Am J Gastroenterol. 2020;115(1):S1224–S1224. https://doi.org/10.14309/01.ajg.0000711304.18292.ad.
Wang S, et al. The efficacy of fecal microbiota transplantation in experimental autoimmune encephalomyelitis: transcriptome and gut microbiota profiling. J Immunol Res. 2021;2021:1–12. https://doi.org/10.1155/2021/4400428.
Li K, et al. Protection of fecal microbiota transplantation in a mouse model of multiple sclerosis. Mediators Inflamm. 2020;2020:1–13. https://doi.org/10.1155/2020/2058272.
Segal A, Zlotnik Y, Moyal-Atias K, Abuhasira R, Ifergane G. Fecal microbiota transplant as a potential treatment for Parkinson’s disease – a case series. Clin Neurol Neurosurg. 2021;207:106791. https://doi.org/10.1016/j.clineuro.2021.106791.
Huang C, et al. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: an EXPLORER trial. J Autoimmun. 2022;130:102844. https://doi.org/10.1016/j.jaut.2022.102844.
Del Negro I, et al. Impact of disease-modifying therapies on gut–brain axis in multiple sclerosis. Med (B Aires). 2023;60(1):6. https://doi.org/10.3390/medicina60010006.
Jangi S, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7(1):12015. https://doi.org/10.1038/ncomms12015.
Katz Sand I, et al. Disease-modifying therapies alter gut microbial composition in MS. Neurol Neuroimmunol Neuroinflamm. 2019;6:1. https://doi.org/10.1212/NXI.0000000000000517.
Ferri C, et al. Gut microbiota changes during dimethyl fumarate treatment in patients with multiple sclerosis. Int J Mol Sci. 2023;24(3):2720. https://doi.org/10.3390/ijms24032720.
Manai F, et al. Dimethyl fumarate and intestine: from main suspect to potential ally against gut disorders. Int J Mol Sci. 2023;24(12):9912. https://doi.org/10.3390/ijms24129912.
Rumah KR, Vartanian TK, Fischetti VA. Oral multiple sclerosis drugs inhibit the in vitro growth of epsilon toxin producing gut bacterium, Clostridium perfringens. Front Cell Infect Microbiol. 2017;7:11. https://doi.org/10.3389/fcimb.2017.00011.
Troci A, et al. B-cell-depletion reverses dysbiosis of the microbiome in multiple sclerosis patients. Sci Rep. 2022;12(1):3728. https://doi.org/10.1038/s41598-022-07336-8.
Tsai C-C, Jette S, Tremlett H. Disease-modifying therapies used to treat multiple sclerosis and the gut microbiome: a systematic review. J Neurol. 2023. https://doi.org/10.1007/s00415-023-12107-0.
Author information
Authors and Affiliations
Contributions
P.O., K.B., A.C.-J., and K.K. contributed to the conceptualization of the manuscript; P.O., K.B., and K.K. analyzed data and drafted the work, and A.C.-J. and K.K. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olejnik, P., Buczma, K., Cudnoch-Jędrzejewska, A. et al. Involvement of gut microbiota in multiple sclerosis—review of a new pathophysiological hypothesis and potential treatment target. Immunol Res (2024). https://doi.org/10.1007/s12026-024-09471-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12026-024-09471-y