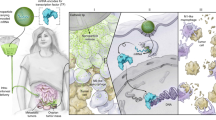
Abstract
An immunosuppressive tumor microenvironment (TME) leads to cancer growth, metastasis, and therapeutic resistance. Immunomodulatory immunotherapy aims to skew the immunosuppressive TME back to an immune active state. Tumor-associated macrophages (TAMs) are a critical component of the TME that are actively involved in tumor-specific inflammation and immunosuppression. TAMs exhibit a diverse range of phenotypes and functions, from pro-tumor to anti-tumor. The plasticity of TAMs makes them a promising target for immunotherapy, and TAM-targeted therapies via different strategies have shown great potential. This review discusses current TAM-specific delivery targets and genes of interest for TAM-reprogramming. As phagocytic cells, TAMs have several receptors that have been used to increase TAM-targeted in vivo delivery. Furthermore, a promising approach for reprogramming TAMs is to activate or suppress specific transcription factors in the signal transducers and activators of transcription (STAT) and interferon regulatory factor (IRF) families. Altering TAM transcription factor expression results in a potent shift in cytokine expression and overall TAM function potentially tipping the balance from an immunosuppressive to an immune active TME.

Similar content being viewed by others
References
Martinez FO, Gordon S. 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Reports. https://doi.org/10.12703/P6-13.
Chávez-Galán L, Olleros ML, Vesin D, Garcia I. Much more than M1 and M2 macrophages, there are also CD169+ and TCR+ macrophages. Front Immunol 2015;6:263. https://doi.org/10.3389/FIMMU.2015.00263.
Hirayama D, Iida T, Nakase H. 2018. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. International Journal of Molecular Sciences 19(92). https://doi.org/10.3390/IJMS19010092.
Duque GA, Descoteaux A. 2014. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5(491). https://doi.org/10.3389/FIMMU.2014.00491/.
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 2013;229(2):176–185. https://doi.org/10.1002/PATH.4133.
Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL. Macrophages: an inflammatory link between angiogenesis and lymphangiogenesis. Microcirculation. 2016;23:95–121.
Ardura JA, Rackov G, Izquierdo E, Alonso V, Gortazar AR, Escribese MM. Targeting macrophages: friends or foes in disease? Front Pharmacol. 2019;10:1255 https://doi.org/10.3389/FPHAR.2019.01255/BIBTEX.
Wang Y, Lin Y-X, Qiao S-L, An H-W, Ma Y, Qiao Z-Y, Rajapaksha RPYJ, Wang H. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials 2017;112:153–163. https://doi.org/10.1016/j.biomaterials.2016.09.034.
Chew V, Toh HC, Abastado JP. 2012. Immune microenvironment in tumor progression: characteristics and challenges for therapy. Journal of Oncology 2012(608406). https://doi.org/10.1155/2012/608406.
Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X. Tumor-associated macrophages: recent insights and therapies. Front Oncol 2020;10:188. https://doi.org/10.3389/FONC.2020.00188/BIBTEX.
Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014;6:1670–1690. https://doi.org/10.3390/cancers6031670.
Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, Ponsoda S, Delneste Y, Hebbar M, Jeannin P. Interferon-γ reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer 2009;125(2):367–373. https://doi.org/10.1002/ijc.24401.
Bardi GT, Smith MA, Hood JL. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine 2018;105:63–72. https://doi.org/10.1016/j.cyto.2018.02.002.
Jayasingam SD, Citartan M, Thang TH, Mat Zin AA, Ang KC, Ch’ng ES. 2020. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Frontiers in Oncology 9(1512). https://doi.org/10.3389/FONC.2019.01512.
Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 2013;73(3):1128–1141. https://doi.org/10.1158/0008-5472.CAN-12-2731.
Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and T H1-TH17 responses. Nature Immunol 2011;12(3):231–238. https://doi.org/10.1038/ni.1990.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nature Rev Clin Oncol 2017;14(7):399–416. https://doi.org/10.1038/nrclinonc.2016.217.
Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J, Ouakrim H, Régnier F, Lupo A, Alifano M, Damotte D, Donnadieu E. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti–PD-1 treatment. Proceedings of the National Academy of Sciences of the United States of America 2018;115 (17):4041–4050. https://doi.org/10.1073/pnas.1720948115.
Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjällman A, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. British J Cancer 2006;95:272–281. https://doi.org/10.1038/sj.bjc.6603240.
Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda SI, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter RM, Ishibashi T, Kuwano M, Ono M. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1β-induced neovascularization and tumor growth. J Clin Investig 2005; 115 (11): 2979–2991. https://doi.org/10.1172/JCI23298.
Macciò A, Gramignano G, Cherchi MC, Tanca L, Melis L, Madeddu C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Scientific Reports 2020;10(1):1–8. https://doi.org/10.1038/s41598-020-63276-1.
Pinto ML, Rios E, Durães C, Ribeiro R, Machado JC, Mantovani A, Barbosa MA, Carneiro F, Oliveira MJ. 2019. The two faces of tumor-associated macrophages and their clinical significance in colorectal cancer. Frontiers in Immunology 10(1875). https://doi.org/10.3389/fimmu.2019.01875.
Conway EM, Pikor LA, Kung SHY, Hamilton MJ, Lam S, Lam WL, Bennewith KL. Macrophages, inflammation, and lung cancer. American Journal of Respiratory and Critical Care Medicine 2016;193(2):116–130. https://doi.org/10.1164/rccm.201508-1545CI.
Lum HD, Buhtoiarov IN, Schmidt BE, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. Tumoristatic effects of anti-CD40 mAb-activated macrophages involve nitric oxide and tumour necrosis factor-α. Immunology 2006;118(2):261–270. https://doi.org/10.1111/J.1365-2567.2006.02366.X.
Wanderley CW, Colón DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA, Pereira JA, Silva CM, Silva CR, Silva RL, Speck-Hernandez CA, Mota JM, Alves-Filho JC, Lima-Junior RC, Cunha TM, Cunha FQ. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res 2018;78(20): 5891–5900. https://doi.org/10.1158/0008-5472.CAN-17-3480.
De Graaff P, Berrevoets C, Rösch C., Schols HA, Verhoef K, Wichers HJ, Debets R, Govers C. Curdlan, zymosan and a yeast-derived β-glucan reshape tumor-associated macrophages into producers of inflammatory chemo-attractants. Cancer Immunology, Immunotherapy 2021;70:547–561. https://doi.org/10.1007/s00262-020-02707-4.
Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology 2012;138:93–104. https://doi.org/10.1111/IMM.12023.
Josephs DH, Bax HJ, Karagiannis SN. Tumour-associated macrophage polarisation and re-education with immunotherapy. Front Biosci 2015;7:334–351. https://doi.org/10.2741/E735.
Genard G, Lucas S, Michiels C. 2017. Reprogramming of tumor-associated macrophages with anticancer therapies: radiotherapy versus chemo- and immunotherapies. https://doi.org/10.3389/fimmu.2017.00828.
Puthenveetil A, Dubey S. Metabolic reprograming of tumor-associated macrophages. Ann Transl Med 2020;8(16):1030–1030. https://doi.org/10.21037/ATM-20-2037.
de Groot AE, Pienta KJ, de Groot AE, Pienta KJ, de Groot AE, Pienta KJ. Epigenetic control of macrophage polarization: implications for targeting tumor-associated macrophages. Oncotarget 2018; 9(29):20908–20927. https://doi.org/10.18632/oncotarget.24556.
Larionova I, Kazakova E, Patysheva M, Kzhyshkowska J. Transcriptional, epigenetic and metabolic programming of tumor-associated macrophages. Cancers 2020;12(6):1411. https://doi.org/10.3390/CANCERS12061411.
Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodríguez-Ruiz ME, Ponz-Sarvise M, Castañón E, Melero I. Cytokines in clinical cancer immunotherapy. British J Cancer 2018;120(1):6–15. https://doi.org/10.1038/s41416-018-0328-y.
Cen X, Liu S, Cheng K. 2018. The role of toll-like receptor in inflammation and tumor immunity. Frontiers in Pharmacology 9(878). https://doi.org/10.3389/fphar.2018.00878.
Niu M, Naguib YW, Aldayel AM, Shi Y-C, Hursting SD, Hersh MA, Cui Z. Biodistribution and in vivo activities of tumor-associated macrophage-targeting nanoparticles incorporated with doxorubicin. Molecular Pharm. 2014;11(12):4425–36 https://doi.org/10.1021/mp500565q.
Han S, Wang W, Wang S, Yang T, Zhang G, Wang D, Ju R, Lu Y, Wang H, Wang L. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics 2021;11(6):2892–2916. https://doi.org/10.7150/thno.50928.
Zhang Y, Wu L, Li Z, Zhang W, Luo F, Chu Y, Chen G. Glycocalyx-mimicking nanoparticles improve anti-pd-l1 cancer immunotherapy through reversion of tumor-associated macrophages. Biomacromolecules 2018;19(6):2098–2108. https://doi.org/10.1021/ACS.BIOMAC.8B00305/SUPPL_FILE/BM8B00305_SI_001.PDF.
Milone MC, O’Doherty U. Clinical use of lentiviral vectors. Leukemia 2018;32(7):1529–1541. https://doi.org/10.1038/s41375-018-0106-0.
Poltavets AS, Vishnyakova PA, Elchaninov AV, Sukhikh GT, Fatkhudinov TK. Macrophage modification strategies for efficient cell therapy. Cells 9(6) (2020). https://doi.org/10.3390/CELLS9061535.
Doronin K, Flatt JW, Di Paolo NC, Khare R, Kalyuzhniy O, Acchione M, Sumida JP, Ohto U, Shimizu T, Akashi-Takamura S, Miyake K, MacDonald JW, Bammler TK, Beyer RP, Farin FM, Stewart PL, Shayakhmetov DM. Coagulation factor X activates innate immunity to human species C adenovirus. Science 2012;338(6108):795–798. https://doi.org/10.1126/SCIENCE.1226625/SUPPL_FILE/DORONIN.SM.PDF.
Takano S, Aramaki Y, Tsuchiya S. Physicochemical properties of liposomes affecting apoptosis induced by cationic liposomes in macrophages. Pharm Res 2003;20(7):962–968. https://doi.org/10.1023/A:1024441702398.
Barnes BJ, Somerville CC. Modulating cytokine production via select packaging and secretion from extracellular vesicles. Front Immunol 2020;11:1040. https://doi.org/10.3389/FIMMU.2020.01040/BIBTEX.
Harrison EB, Azam SH, Pecot CV. Targeting accessories to the crime: nanoparticle nucleic acid delivery to the tumor microenvironment. Front Pharmacol. 2018;9:307 https://doi.org/10.3389/fphar.2018.00307.
Hu G, Guo M, Xu J, Wu F, Fan J, Huang Q, Yang G, Lv Z, Wang X, Jin Y. 2019. Nanoparticles targeting macrophages as potential clinical therapeutic agents against cancer and inflammation. Frontiers in Immunology 10(1998). https://doi.org/10.3389/FIMMU.2019.01998.
Dehaini D, Fang RH, Zhang L. Biomimetic strategies for targeted nanoparticle delivery. Bioeng Transl Med 2016;1:30–46. https://doi.org/10.1002/btm2.10004.
Pei Y, Yeo Y. Drug delivery to macrophages: challenges and opportunities. J Control Release. 2016;240:202–11 https://doi.org/10.1016/J.JCONREL.2015.12.014.
Rao L, Zhao SK, Wen C, Tian R, Lin L, Cai B, Sun Y, Kang F, Yang Z, He L, Mu J, Meng QF, Yao G, Xie N, Chen X. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv Mater 2020;32(47):2004853. https://doi.org/10.1002/ADMA.202004853.
Medina TP, Gerle M, Humbert J, Chu H, Köpnick AL, Barkmann R, Garamus VM, Sanz B, Purcz N, Will O, Appold L, Damm T, Suojanen J, Arnold P, Lucius R, Willumeit-Römer R, Açil Y, Wiltfang J, Goya GF, Glüer CC, Medina OP. Lipid-iron nanoparticle with a cell stress release mechanism combined with a local alternating magnetic field enables site-activated drug release. Cancers 2020;12(12):3767. https://doi.org/10.3390/CANCERS12123767.
Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, Holland EC, Stephan MT. 2019. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nature Communications 10(3974). https://doi.org/10.1038/s41467-019-11911-5.
Shi C, Liu T, Guo Z, Zhuang R, Zhang X, Chen X. Reprogramming tumor-associated macrophages by nanoparticle-based reactive oxygen species photogeneration. Nano Lett 2018;18(11): 7330–7342. https://doi.org/10.1021/ACS.NANOLETT.8B03568/SUPPL_FILE/NL8B03568_SI_004.XLSX.
Liu C.-P., Zhang X, Tan Q.-L., Xu W.-X., Zhou C.-Y., Luo M, Li X, Huang R.-Y., Zeng X. NF-κ B pathways are involved in M1 polarization of RAW 264.7 macrophage by polyporus polysaccharide in the tumor microenvironment. PLOS ONE 2017;12(11):0188317. https://doi.org/10.1371/JOURNAL.PONE.0188317.
Da Silva CG, Camps MGM, Li TMWY, Chan AB, Ossendorp F, Cruz LJ. Co-delivery of immunomodulators in biodegradable nanoparticles improves therapeutic efficacy of cancer vaccines. Biomaterials 2019;220:119417. https://doi.org/10.1016/J.BIOMATERIALS.2019.119417.
Ortega RA, Barham WJ, Kumar B, Tikhomirov O, McFadden ID, Yull FE, Giorgio TD. Biocompatible mannosylated endosomal-escape nanoparticles enhance selective delivery of short nucleotide sequences to tumor associated macrophages. Nanoscale 2014;7(2):500–510. https://doi.org/10.1039/C4NR03962A.
Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L, Yu X, Luo Q, Zhang Z. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11(9):9536–49 https://doi.org/10.1021/ACSNANO.7B05465/SUPPL_FILE/NN7B05465_SI_001.PDF.
Georgoudaki A-M, Prokopec K, Boura V, Hellqvist E, Sohn S, Östling J, Dahan R, Harris R, Rantalainen M, Klevebring D, Sund M, Brage S, Fuxe J, Rolny C, Li F, Ravetch J, Karlsson MI. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Reports. 2016;15(9):2000–11 https://doi.org/10.1016/J.CELREP.2016.04.084.
Etzerodt A, Maniecki MB, Graversen JH, Moller HJ, Torchilin VP, Moestrup SK. Efficient intracellular drug-targeting of macrophages using stealth liposomes directed to the hemoglobin scavenger receptor CD163. J Control Release 2012;160(1):72–80. https://doi.org/10.1016/J.JCONREL.2012.01.034.
Zhuang H, Dai X, Zhang X, Mao Z, Huang H. Sophoridine suppresses macrophage-mediated immunosuppression through TLR4/IRF3 pathway and subsequently upregulates CD8+ T cytotoxic function against gastric cancer. Biomed Pharmacother 2020;121:109636. https://doi.org/10.1016/J.BIOPHA.2019.109636.
Su L, Zhang W, Wu X, Zhang Y, Chen X, Liu G, Chen G, Jiang M. Glycocalyx-mimicking nanoparticles for stimulation and polarization of macrophages via specific interactions. Small 2015;11(33): 4191–4200. https://doi.org/10.1002/smll.201403838.
Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SYC, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med 2002;196(3): 407–412. https://doi.org/10.1084/JEM.20020470.
Zhang M, Gao Y, Caja K, Zhao B, Kim J. Non-viral nanoparticle delivers small interfering RNA to macrophages in vitro and in vivo. PLoS ONE. 2015;10(3):0118472 https://doi.org/10.1371/JOURNAL.PONE.0118472.
Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nature Rev Immunol 2013;13(9):621–634. https://doi.org/10.1038/nri3515.
Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, Xu D. 2020. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Frontiers in Immunology 11(1731). https://doi.org/10.3389/FIMMU.2020.01731.
Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nature Rev Immunol 2015;15(7):405–414. https://doi.org/10.1038/nri3845.
Wang N, Liang H, Zen K. 2014. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Frontiers in Immunology 5(614). https://doi.org/10.3389/fimmu.2014.00614.
Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nature Immunol 2017;18(4):374–384. https://doi.org/10.1038/ni.3691.
Platanitis E, Decker T. 2018. Regulatory networks involving STATs, IRFs, and NFκ B in inflammation. Frontiers in Immunology 9(2542). https://doi.org/10.3389/fimmu.2018.02542.
Kim HS, Kim DC, Kim HM, Kwon HJ, Kwon SJ, Kang SJ, Kim SC, Choi GE. STAT1 deficiency redirects IFN signalling toward suppression of TLR response through a feedback activation of STAT3. Scient Rep 2015;5(1):1–15. https://doi.org/10.1038/srep13414.
Hu X, Ivashkiv LB. Cross-regulation of signaling and immune responses by IFN-γ and STAT1. Immunity. 2009;31(4):539 https://doi.org/10.1016/J.IMMUNI.2009.09.002.
Mancino A, Lawrence T. Nuclear factor-κ B and tumor-associated macrophages. Clinical Cancer Res 2010; 16 (3): 784–789. https://doi.org/10.1158/1078-0432.CCR-09-1015.
Frank DA, Robertson MJ, Bonni A, Ritz J, Greenberg ME. Interleukin 2 signaling involves the phosphorylation of Stat proteins. Proceedings of the National Academy of Sciences of the United States of America 1995;92(17):7779. https://doi.org/10.1073/PNAS.92.17.7779.
Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature Rev Immunol 2011;11(11):750–761. https://doi.org/10.1038/nri3088.
Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol 2013;33(6):1135–1144. https://doi.org/10.1161/ATVBAHA.113.301453.
Budhwani M, Mazzieri R, Dolcetti R. Plasticity of type I interferon-mediated responses in cancer therapy: from anti-tumor immunity to resistance. Front Oncol 2018;8:322. https://doi.org/10.3389/fonc.2018.00322.
Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol 2005;174(8):4880–4891. https://doi.org/10.4049/JIMMUNOL.174.8.4880.
Petrillo M, Zannoni GF, Martinelli E, Anchora LP, Ferrandina G, Tropeano G, Fagotti A, Scambia G. Polarisation of tumor-associated macrophages toward m2 phenotype correlates with poor response to chemoradiation and reduced survival in patients with locally advanced cervical cancer. PLoS ONE 2015; 10(9):0136654. https://doi.org/10.1371/JOURNAL.PONE.0136654.
Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-κ B. J Exp Med 2008;205(6):1261–1268. https://doi.org/10.1084/jem.20080108.
Tymoszuk P, Evens H, Marzola V, Wachowicz K, Wasmer MH, Datta S, Müller-Holzner E, Fiegl H, Böck G, van Rooijen N, Theurl I, Doppler W. In situ proliferation contributes to accumulation of tumor-associated macrophages in spontaneous mammary tumors. Eur J Immunol 2014; 44(8):2247–2262. https://doi.org/10.1002/EJI.201344304.
Iriki T, Ohnishi K, Fujiwara Y, Horlad H, Saito Y, Pan C, Ikeda K, Mori T, Suzuki M, Ichiyasu H, Kohrogi H, Takeya M, Komohara Y. The cell-cell interaction between tumor-associated macrophages and small cell lung cancer cells is involved in tumor progression via STAT3 activation. Lung Cancer 2017;106:22–32. https://doi.org/10.1016/J.LUNGCAN.2017.01.003.
Zhang X, Tian W, Cai X, Wang X, Dang W, Tang H, Cao H, Wang L, Chen T. Hydrazinocurcumin encapsuled nanoparticles “Re-Educate” tumor-associated macrophages and exhibit anti-tumor effects on breast cancer following STAT3 suppression. PLoS ONE 2013;8(6):65896. https://doi.org/10.1371/JOURNAL.PONE.0065896.
Binnemars-Postma K, Bansal R, Storm G, Prakash J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J 2018;32 (2):969–978. https://doi.org/10.1096/fj.201700629R.
Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clément K, Jain MK. Krüppel-like factor 4 regulates macrophage polarization. J Clin Investig 2011;121(7):2736. https://doi.org/10.1172/JCI45444.
Xiao H, Guo Y, Li B, Li X, Wang Y, Han S, Cheng D, Shuai X. M2-Like tumor-associated macrophage-targeted codelivery of STAT6 inhibitor and IKKβ siRNA induces M2-to-M1 repolarization for cancer immunotherapy with low immune side effects. ACS Central Sci 2020;6(7):1208–1222. https://doi.org/10.1021/acscentsci.9b01235.
Günthner R, Anders H-J. Interferon-regulatory factors determine macrophage phenotype polarization. Mediat Inflamm 2013;2013:1–8. https://doi.org/10.1155/2013/731023.
Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem 2007; 282(21):15325–9. https://doi.org/10.1074/jbc.R700002200.
Romieu-Mourez R, Solis M, Nardin A, Goubau D, Baron-Bodo V, Lin R, Massie B, Salcedo M, Hiscott J. Distinct roles for IFN regulatory factor (IRF)-3 and IRF-7 in the activation of antitumor properties of human macrophages. Cancer Res 2006;66(21):10576–10585. https://doi.org/10.1158/0008-5472.CAN-06-1279.
Wang F, Gao X, Barrett JW, Shao Q, Bartee E, Mohamed MR, Rahman M, Werden S, Irvine T, Cao J, Dekaban GA, McFadden G. RIG-I mediates the co-induction of tumor necrosis factor and type I interferon elicited by myxoma virus in primary human macrophages. PLoS Pathogens 2008;4 (7):1000099. https://doi.org/10.1371/journal.ppat.1000099.
Chiang H-S, Liu HM. The molecular basis of viral inhibition of IRF- and STAT-dependent immune responses. Front Immunol. 2019;1:3086 https://doi.org/10.3389/fimmu.2018.03086.
Chistiakov DA, Myasoedova VA, Revin VV, Orekhov AN, Bobryshev YV. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018;223(1):101–111. https://doi.org/10.1016/J.IMBIO.2017.10.005.
Zhang X, Artola-Boran M, Fallegger A, Arnold IC, Weber A, Reuter S, Taube C, Müller A. IRF4 expression is required for the immunoregulatory activity of conventional type 2 dendritic cells in settings of chronic bacterial infection and cancer. J Immunol. 2020;205(7):1933–43 https://doi.org/10.4049/JIMMUNOL.2000405/-/DCSUPPLEMENTAL.
Balkhi MY, Fitzgerald KA, Pitha PM. Functional regulation of MyD88-activated interferon regulatory factor 5 by K63-linked polyubiquitination. Mol Cell Biol 2008;28(24):7296–7308. https://doi.org/10.1128/MCB.00662-08.
Chen W, Lam SS, Srinath H, Jiang Z, Correia JJ, Schiffer CA, Fitzgerald KA, Lin K, Royer WE. Insights into interferon regulatory factor activation from the crystal structure of dimeric IRF5. Nat Struct Mol Biol 2008;15(11):1213–1220. https://doi.org/10.1038/nsmb.1496.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Musick, M., Yu, X. Manipulation of the tumor immuno-microenvironment via TAM-targeted expression of transcription factors. Immunol Res 70, 432–440 (2022). https://doi.org/10.1007/s12026-022-09277-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09277-w