Abstract
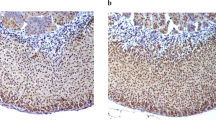
Ubiquitin-specific protease 10 (USP10), a novel deubiquitinating enzyme, is associated with androgen receptor transcriptional activity and pathological processes of tumor. However, information between USP10 and the adrenal gland is limited. In particular, the role of USP10 in adrenal tumors has not been elucidated yet. This study aims to investigate the expression of USP10 in the human normal adrenal gland and various adrenal tumors. Tissue samples were obtained from 30 adrenocortical adenomas, nine adrenocortical adenocarcinomas, and 20 pheochromocytomas following laparoscopic surgery. Twenty normal adrenal glands were obtained from kidney surgical resection conducted due to renal cell carcinomas. USP10 expression was investigated on protein levels using immunohistochemistry and on mRNA levels using bioinformatics analysis in the Gene Expression Omnibus (GEO) Datasets. In the 20 cases of normal adrenal glands analyzed, USP10 protein was constantly expressed in situ in the cortex of the adrenal glands, but in the medulla of the gland, only the sustentacular cells were detected positive. In adrenal tumors, detectable levels of USP10 protein were found in 100 % (30/30) adrenocortical adenomas, 88.89 % (8/9) adrenocortical carcinomas, and 10 % (2/20) pheochromocytomas. Bioinformatics analysis did not show a significant difference in USP10 messenger RNA (mRNA) expression between adrenal tumors and normal adrenal gland tissues. A positive USP10 immunoreaction can be useful in distinguishing adrenal cortical tumors from pheochromocytoma.





Similar content being viewed by others
References
Soncini C, Berdo I, Draetta G (2001) Ras-GAP SH3 domain binding protein (G3BP) is a modulator of USP10, a novel human ubiquitin specific protease. Oncogene 20 (29):3869-3879. doi:10.1038/sj.onc.1204553
Pan L, Chen Z, Wang L, Chen C, Li D, Wan H, Li B, Shi G (2014) Deubiquitination and stabilization of T-bet by USP10. Biochem Biophys Res Commun 449 (3):289-294. doi:10.1016/j.bbrc.2014.05.037
Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Norberg HV, Zhang T, Furuya T, Jin M, Zhu Z, Wang H, Yu J, Hao Y, Choi A, Ke H, Ma D, Yuan J (2011) Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 147 (1):223-234. doi:10.1016/j.cell.2011.08.037
Deng S, Zhou H, Xiong R, Lu Y, Yan D, Xing T, Dong L, Tang E, Yang H (2007) Over-expression of genes and proteins of ubiquitin specific peptidases (USPs) and proteasome subunits (PSs) in breast cancer tissue observed by the methods of RFDD-PCR and proteomics. Breast Cancer Res Treat 104 (1):21-30. doi:10.1007/s10549-006-9393-7
Grunda JM, Nabors LB, Palmer CA, Chhieng DC, Steg A, Mikkelsen T, Diasio RB, Zhang K, Allison D, Grizzle WE, Wang W, Gillespie GY, Johnson MR (2006) Increased expression of thymidylate synthetase (TS), ubiquitin specific protease 10 (USP10) and survivin is associated with poor survival in glioblastoma multiforme (GBM). J Neurooncol 80 (3):261-274. doi:10.1007/s11060-006-9191-4
Zeng Z, Wu HX, Zhan N, Huang YB, Wang ZS, Yang GF, Wang P, Fu GH (2014) Prognostic significance of USP10 as a tumor-associated marker in gastric carcinoma. Tumour Biol 35 (4):3845-3853. doi:10.1007/s13277-013-1509-1
Yuan J, Luo K, Zhang L, Cheville JC, Lou Z (2010) USP10 regulates p53 localization and stability by deubiquitinating p53. Cell 140 (3):384-396. doi:10.1016/j.cell.2009.12.032
Liu H, Xu XF, Zhao Y, Tang MC, Zhou YQ, Lu J, Gao FH (2014) MicroRNA-191 promotes pancreatic cancer progression by targeting USP10. Tumour Biol 35 (12):12157-12163. doi:10.1007/s13277-014-2521-9
Bentvelsen FM, McPhaul MJ, Wilson CM, Wilson JD, George FW (1996) Regulation of immunoreactive androgen receptor in the adrenal gland of the adult rat. Endocrinology 137 (7):2659-2663. doi:10.1210/endo.137.7.8770883
Faus H, Meyer HA, Huber M, Bahr I, Haendler B (2005) The ubiquitin-specific protease USP10 modulates androgen receptor function. Mol Cell Endocrinol 245 (1-2):138-146. doi:S0303-7207(05)00405-3
Draker R, Sarcinella E, Cheung P (2011) USP10 deubiquitylates the histone variant H2A.Z and both are required for androgen receptor-mediated gene activation. Nucleic Acids Res 39 (9):3529-3542. doi:10.1093/nar/gkq1352
Aubert S, Wacrenier A, Leroy X, Devos P, Carnaille B, Proye C, Wemeau JL, Lecomte-Houcke M, Leteurtre E (2002) Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol 26 (12):1612-1619
Ge X, Yamamoto S, Tsutsumi S, Midorikawa Y, Ihara S, Wang SM, Aburatani H (2005) Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 86 (2):127-141. doi:S0888-7543(05)00111-4
Dezso Z, Nikolsky Y, Sviridov E, Shi W, Serebriyskaya T, Dosymbekov D, Bugrim A, Rakhmatulin E, Brennan RJ, Guryanov A, Li K, Blake J, Samaha RR, Nikolskaya T (2008) A comprehensive functional analysis of tissue specificity of human gene expression. BMC Biol 6:49. doi:10.1186/1741-7007-6-49
Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G (2009) Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res 15 (2):668-676. doi:10.1158/1078-0432.CCR-08-1067
Giubellino A, Shankavaram U, Bullova P, Schovanek J, Zhang Y, Shen M, Patel N, Elkahloun A, Lee MJ, Trepel J, Ferrer M, Pacak K (2014) High-throughput screening for the identification of new therapeutic options for metastatic pheochromocytoma and paraganglioma. PLoS One 9 (4):e90458. doi:10.1371/journal.pone.0090458
Cheng LL, Itahana Y, Lei ZD, Chia NY, Wu Y, Yu Y, Zhang SL, Thike AA, Pandey A, Rozen S, Voorhoeve PM, Yu Q, Tan PH, Bay BH, Itahana K, Tan P (2012) TP53 genomic status regulates sensitivity of gastric cancer cells to the histone methylation inhibitor 3-deazaneplanocin A (DZNep). Clin Cancer Res 18 (15):4201-4212. doi:10.1158/1078-0432.CCR-12-0036
Bomberger JM, Barnaby RL, Stanton BA (2010) The deubiquitinating enzyme USP10 regulates the endocytic recycling of CFTR in airway epithelial cells. Channels (Austin) 4 (3):150-154. doi:11223
Bomberger JM, Barnaby RL, Stanton BA (2009) The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem 284 (28):18778-18789. doi:10.1074/jbc.M109.001685
Lin Z, Yang H, Tan C, Li J, Liu Z, Quan Q, Kong S, Ye J, Gao B, Fang D (2013) USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep 5 (6):1639-1649. doi:10.1016/j.celrep.2013.11.029
Takahashi M, Higuchi M, Makokha GN, Matsuki H, Yoshita M, Tanaka Y, Fujii M (2013) HTLV-1 Tax oncoprotein stimulates ROS production and apoptosis in T cells by interacting with USP10. Blood 122 (5):715-725. doi:10.1182/blood-2013-03-493718
Niu J, Shi Y, Xue J, Miao R, Huang S, Wang T, Wu J, Fu M, Wu ZH (2013) USP10 inhibits genotoxic NF-kappaB activation by MCPIP1-facilitated deubiquitination of NEMO. EMBO J 32 (24):3206-3219. doi:10.1038/emboj.2013.247
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81571147 and 81301019) and Independent Scientific Research Subject for Young Teachers of Wu Han University (Grant No. 2042015kf0076).
Authors’ Contribution
Zeng Z: Project development and manuscript writing
Zhou ZY, Zhan N, Yuan JP, and Ye BX: Data collection or management
Gu LJ, Wang J, and Jian ZH: Data analysis
Xiong XX: Protocol development and manuscript editing
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of Renmin Hospital of the Wuhan University and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zeng, Z., Zhou, Z., Zhan, N. et al. USP10 Expression in Normal Adrenal Gland and Various Adrenal Tumors. Endocr Pathol 26, 302–308 (2015). https://doi.org/10.1007/s12022-015-9406-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-015-9406-3