Abstract
Introduction
Roux-en-Y gastric bypass (RYGB) leads to beneficial effects on glucose homeostasis, and attenuated hormonal counterregulatory responses to hypoglycemia are likely to contribute. RYGB also induces alterations in neural activity of cortical and subcortical brain regions. We aimed to characterize RYGB-induced changes in resting-state connectivity of specific brain regions of interest for energy homeostasis and behavioral control during hypoglycemia.
Method
Ten patients with BMI > 35 kg/m2 were investigated with brain PET/MR imaging during a hyperinsulinemic normo- and hypoglycemic clamp, before and 4 months after RYGB. Hormonal levels were assessed throughout the clamp. Resting-state (RS) fMRI scans were acquired in the glucose-lowering phase of the clamp, and they were analyzed with a seed-to-voxel approach.
Results
RS connectivity during initiation of hypoglycemia was significantly altered after RYGB between nucleus accumbens, thalamus, caudate, hypothalamus and their crosstalk with cortical and subcortical regions. Connectivity between the nucleus accumbens and the frontal pole was increased after RYGB, and this was associated with a reduction of ACTH (r = −0.639, p = 0.047) and cortisol (r = −0.635, p = 0.048) responses. Instead, connectivity between the caudate and the frontal pole after RYGB was reduced and this was associated with less attenuation of glucagon response during the hypoglycemic clamp (r = −0.728, p = 0.017), smaller reduction in fasting glucose (r = −0.798, p = 0.007) and less excess weight loss (r = 0.753, p = 0.012). No other significant associations were found between post-RYGB changes in ROI-to-voxel regional connectivity hormonal responses and metabolic or anthropometric outcomes.
Conclusion
RYGB alters brain connectivity during hypoglycemia of several neural pathways involved in reward, inhibitory control, and energy homeostasis. These changes are associated with altered hormonal responses to hypoglycemia and may be involved in the glucometabolic outcome of RYGB.
Similar content being viewed by others
Introduction
Roux-en-Y gastric bypass (RYGB) is a surgical procedure that induces durable weight loss and has a positive impact on glucose homeostasis, lowering the risk of type 2 diabetes or even leading to diabetes reversal [1, 2]. This is achieved via gut anatomical and physiological rearrangements including hormonal adaptations that lead to enhanced incretin secretion and improved beta-cell function [3]. However, intriguing evidence suggests a much-underexplored role of the central nervous system in mediating the favorable effects of bariatric surgery [4].
Energy metabolism and food intake are finely regulated processes that rely on the hypothalamic integration of both central and peripheral signals [5]. However, the mesolimbic dopamine pathway (also known as the brain reward system) has been recently more and more recognized for its role in regulating glucose homeostasis [6], likely also via crosstalk with peripheral hormonal signals, including glucagon-like peptide 1 (GLP-1), insulin, ghrelin, and leptin [7,8,9].
The brain reward system is in engaged in the consumption of palatable food [10, 11], as well as by drugs of abuse [12]. Therefore, it is likely that aberrant eating behavior leading to obesity is related to morpho-functional abnormalities in the reward system neurocircuitry [11, 13, 14].
Neuroimaging techniques are versatile, non-invasive informative procedures and have highlighted changes in brain functional connectivity and glucose metabolism upon bariatric intervention. Several studies in the latest years have highlighted that after RYGB, resting-state connectivity in the fasting state is reshaped, especially as to brain regions part of the default-mode network (cingulate cortex, precuneus, basal ganglia), the prefrontal cortex, and the cerebellum [15,16,17,18,19]. Also, brain glucose uptake as assessed by 18F-FDG-PET in energy balance regions is altered after RYGB [20].
Our group was the first to perform a combined PET/MRI assessment under a hyperinsulinemic clamp with both a euglycemic and a hypoglycemic phase in patients before and after undergoing RYGB [21]. During the glucose-lowering phase, we found increased resting-state connectivity after surgery in an independent component including bilateral thalamus and hypothalamus, the brain’s central control unit of energy metabolism. Also, in a post-hoc analysis, we found that lateral hypothalamus connectivity was decreased towards the hippocampus and increased to the cerebellar vermis after RYGB [21]. This might reflect an RYGB-induced adaptation in neurocircuitries leading to a dampened response to hypoglycemia.
We hypothesize that brain connectivity after RYGB is reshaped in order to dampen the homeostatic response to energy deprivation. Therefore, in this study, we focused on RYGB-associated changes in regional brain connectivity during the glucose-lowering phase of the hyperinsulinemic clamp. We targeted brain regions that are of relevance for energy homeostasis and eating behavior according to previous work, including the thalamus, the ventral tegmental area, the basal nuclei, the amygdala, and the hypothalamus. The findings support a role of the brain in the favorable metabolic adaptations brought about by bariatric surgery.
Methods
Study design and subjects
Ten non-diabetic individuals with obesity (aged 25–49, BMI 35.2–45.4 kg/m2) were recruited at the obesity outpatient facility at Uppsala University Hospital during a routine visit before the scheduled Roux-en-Y gastric bypass (RYGB) operation. Further details of this cohort have been described and published before [21]. The subjects were investigated twice: approximately one month before RYGB and 4 months after RYGB. Also, according to local intervention guidelines, the patients underwent a 4-week low-calorie diet (800–1100 kcal/day) before surgical intervention. The study visits were performed at the PET/MR facility at Uppsala University Hospital and started at 8 am when the subjects were required to show up after an overnight fast. Medical history, anthropometric measures including total body fat assessed with bioimpedance, and blood samples were obtained.
Investigations
At each visit, the subjects underwent a combined metabolic and neuroimaging assessment with simultaneous PET/MRI imaging and a two-step hyperinsulinemic clamp. This consisted of a first normoglycemic phase (5 mM) and a second hypoglycemic phase (2.7 mM). Also, cognitive assessments were performed during both the normoglycemic and the hypoglycemic phase using the Trail Making Test (TMT) and the Digital Symbol Substitution Test (DSST). Hypoglycemic symptoms were evaluated during hypoglycemia via the Edinburgh hypoglycemia symptoms score (EHSS). Blood samples were drawn from an antecubital vein after arterialization and were analyzed at the Department of Clinical Chemistry and the Clinical Diabetes Research Laboratory of Uppsala University Hospital. Neuroimaging was performed using an integrated PET/MR machine (SIGNA; GE Healthcare, Waukesha, WI), comprising a 3 T MRI scanner [21]. Details describing the steps of the whole combined procedure are described elsewhere [21]. During the clamp investigation, functional MRI (fMRI) scanning was run on three occasions: (1) during the normoglycemic phase; (2) during the glucose-lowering phase, and (3) during the hypoglycemic phase. Neuroimaging postprocessing details are described in supplemental materials of our previous publication [21].
Neuroimaging exploratory analyses
For this post-hoc analysis, the CONN toolbox version 21.a [22] was used to identify changes after RYGB in regional connectivity of pre-determined seed regions of interest (ROI) to the whole brain, using a ROI-to-voxel approach. Anatomical demarcation of ROIs was performed with the embedded anatomic atlas of the CONN toolbox. Chosen seed regions were nucleus accumbens, caudate nucleus, putamen, medial and lateral hypothalamus, thalamus, amygdala, hippocampus, and globus pallidus. The software algorithm performed an automatic false discovery rate (FDR) correction of the results. All analyses were performed by averaging left and right hemispheres.
Statistical analyses
Data are presented as mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate and specifically indicated. Correlation analyses were performed with Pearson’s method after checking for normal distribution of the data using the Shapiro-Wilk test. Significance level was set at p = 0.05. Because of the exploratory nature of this investigation, correlation analyses were not corrected for FDR, and nominal p-values are given. Statistical analyses were performed using GraphPad Prism 9, and images were created using the CONN toolbox [22] and GraphPad Prism 9.
Results
Using a ROI-to-voxel approach, we found several voxel clusters that displayed different connectivity after RYGB with brain regions that are relevant to energy homeostasis and feeding behavior (Table 1).
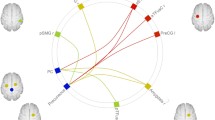
Connectivity of the nucleus accumbens with voxel clusters located in both left and right frontal poles and the right thalamus was increased (Fig. 1A). The caudate nucleus showed increased connectivity with the posterior division of the left middle temporal gyrus, and decreased connectivity with the cortex of the medial surface of brain hemispheres, including anterior and posterior cingulate and paracingulate gyrus, precuneus, and frontal pole (Fig. 1B). Thalamus connectivity was increased with a cluster located on the right side stretching over paleostriatum (globus pallidus), neostriatum (caudate nucleus) and ventral striatum (nucleus accumbens), while decreased with the right cerebellum (Fig. 1C). We found increased putamen connectivity with both right and left cerebellum. Lateral hypothalamus showed the greatest changes in connectivity after RYGB, with altered connectivity with several medial and lateral cortical areas, left hippocampus and parahippocampal gyrus, cerebellum, and the putamen (Fig. 1D). Connectivity of the medial hypothalamus was increased with the cerebellum and decreased with the right superior parietal lobule (Fig. 1E). On the contrary, regional connectivity of amygdala, hippocampus and globus pallidus were unaffected after RYGB (Table 1).
ROI-to-voxel analysis with changes in connectivity after RYGB in the glucose-lowering phase of the hyperinsulinemic clamp. The brain slice images (right part of the panel) show in red the voxel clusters that have significantly different connectivity with the seed region (depicted in blue on the left). A Nucleus accumbens, B caudate nucleus, C thalamus, D lateral hypothalamus, E medial hypothalamus. Created with the CONN toolbox
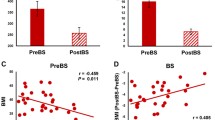
Increased connectivity between the nucleus accumbens and the frontal pole was associated with reduced iAUC (incremental area under the curve) of both ACTH (r = −0.639, p = 0.047) and cortisol (r = −0.635, p = 0.048) during the hypoglycemic clamp (Fig. 2A, B).
However, connectivity between the caudate and the frontal pole after RYGB was reduced and this correlated with less reduction of the glucagon response during the hypoglycemic clamp (r = −0.728, p = 0.017 for iAUC changes; Fig. 2C), smaller reduction of fasting plasma glucose (r = −0.798, p = 0.007; Fig. 2D), as well as with lower percentage excess weight loss (%EWL) (r = 0.753, p = 0.012; Fig. 2E).
None of the other changes in regional connectivity was associated with %EWL, changes in BMI, fasting plasma glucose, waist-to-hip ratio, clamp M-value, body fat percentage, or the ∆iAUC of any other counterregulatory hormone (data not shown).
We have previously shown that cognitive function as assessed by psychometric tests correlated with cerebral blood flow during hypoglycemia, and that both of them were increased after RYGB [21]. However, these changes were not associated with any surgery-induced change in regional connectivity after RYGB (data not shown).
Discussion
We found several resting-state neural pathways whose activity was altered after RYGB during the glucose-lowering phase of the hyperinsulinemic clamp, involving both basal nuclei and cortical regions. Some of these changes were also associated with surgery-induced changes in brain glucose metabolism and peripheral counterregulatory responses.
Striatum and frontal cortex
In the mesolimbic dopamine pathway, the release of dopamine by neurons projecting from the ventral tegmental area onto dopamine receptors expressed by the GABAergic spiny neurons of the nucleus accumbens promotes the desire for rewarding stimuli, a process known as incentive salience [23]. Both short-projecting GABAergic neurons within the ventral tegmental area [24] and long-projecting GABAergic neurons to the shell of the nucleus accumbens [25] participate in the fine-tuning of this network. We found increased connectivity between the nucleus accumbens and the frontal pole, a region whose neurological function is still poorly understood, but seems to be involved in the so-called cognitive branching [26], that is the ability to attribute priority to different tasks scheduled to achieve an objective. Since efferent nucleus accumbens projections are GABAergic, thus inhibitory [27], we speculate that the increased connectivity between these regions during the glucose-lowering phase reflects a reduction in the cognitive response to energy deprivation. In addition, patients with the greatest increase in this connectivity also showed the most attenuated HPA-axis activation following RYGB. Altogether, these findings suggest that RYGB leads to intertwined readaptations of glucose homeostasis at several levels, affecting hormonal and cognitive responses, brain connectivity and glucose metabolism.
The connectivity between the nucleus accumbens and the thalamus, as parts of the reward system, is responsible for the translation of motivation inputs into motor responses [28]. We also found increased connectivity between these two regions after RYGB, suggesting an altered motivational incentive to food-seeking during hypoglycemia.
Connectivity between the caudate nucleus and the frontal pole, which operate together in behavioral inhibitory control [29] and goal-directed behavior [30], might also be relevant in behavioral adaptation to hypoglycemia after RYGB. Remarkably, patients with the greatest attenuations in this connectivity pathway had the least reduction in glucagon responses during the hypoglycemic clamp, less loss of excess weight and less improvement in fasting plasma glucose, suggesting a central compensatory mechanism to the great RYGB-induced metabolic adaptations.
Default-mode network
A reduction in the posterior cingulate cortex and the precuneus functional connectivity is known to occur at fasting after gastric bypass [15,16,17,18]. Both the posterior cingulate cortex and the precuneus are key neural hubs of the default-mode network and play a role in internally directed attention and emotional salience [31, 32]. Deactivation of this region after RYGB is suggested to indicate reduced food-related salience attribution and reward-driven eating behavior [18]. Decreased connectivity of these regions with the caudate nucleus in the hypoglycemic phase suggests reduced salience attribution to the sense of hunger during energy deprivation after RYGB. Moreover, this system could also be directly influenced by homeostatic outputs from the lateral hypothalamus since we found increased connectivity of the latter with the posterior cingulate gyrus and the precuneus.
Cerebellum connectivity
We found decreased connectivity of several brain regions, including caudate nucleus, putamen, medial hypothalamus, and thalamus, with the cerebellum, whilst connectivity of both medial and lateral hypothalamus with the cerebellum was increased. The cerebellum is implicated in excessive eating and satiation signals [33, 34]. Simultaneous transcranial stimulation of the prefrontal cortex and the cerebellum induced remarkable alterations in appetite and desire to eat in human obese individuals [35]. Our results support the role of RYGB in remodeling neurocircuitry between several subcortical regions and the cerebellum during hypoglycemia, in accordance with the emerging evidence that attributes the cerebellum a relevant role in feeding behavior and satiation [34].
Thalamus connectivity
We found altered connectivity between the thalamus and the nucleus accumbens after RYGB. Paraventricular thalamic projections to the nucleus accumbens are important in motivated behaviors [28] and promote both feeding [36] and sucrose-seeking behaviors during hypoglycemia [37], as well as and high-fat diet preference [38] in murine models. Thus, RYGB might affect feeding behavior through modulating motivational aspects of feeding behavior, especially in conditions of energy deprivation, with improved metabolic aftermaths.
We also found increased connectivity of the thalamus with the caudate nucleus and the globus pallidus, a pathway implied in cognitive control on behavior [39].
Hypothalamus connectivity
We found increased connectivity between the lateral hypothalamus and the hippocampus. A study carried out on patients with narcolepsy identified decreased connectivity between the lateral hypothalamus and the hippocampus, and speculated that this was a proxy for decreased orexin/hypocretin innervation, a neurotransmitter that is of great importance also in appetite regulation [40]. This circuitry featuring melanin-concentrating hormone neurotransmission is not only extremely relevant in orexigenesis but is also involved in impulsive behavior [41].
Recently, it has been shown that connectivity between the lateral hypothalamus and several cerebellar regions was associated with the weight loss outcome in obese subjects undergoing a 3-month diet [42]. Accordingly, we found increased connectivity in this pathway after RYGB, suggest its favorable role in weight control.
Limitations
This study has some limitations. Firstly, it is an exploratory post-hoc investigation, so no formal power calculations were made and some of the analyses might therefore be underpowered. Secondly, this is an observational study, and no conclusions of causality can be made based on the presented results. Thirdly, the lack of a control group does not allow to distinguish between the effects of RYGB and the weight loss per se. However, several studies have highlighted the structural and functional changes following bariatric surgery in the human brain being independent of weight loss [43]. Finally, the sample size is small and true changes in other neural pathways may have been undetected due to lack of power as well as due to the ROI selection. Therefore, these findings should be used to generate hypotheses, and they need confirmation in additional studies.
Conclusion
Altogether, these findings highlight profound changes following RYGB in brain cortical and subcortical connectivity during experimental glucose deprivation. The affected neural pathways are largely involved in reward, inhibitory control or energy homeostasis. Among them, increased connectivity between nucleus accumbens and the frontal pole was associated with attenuated counterregulatory hormonal responses to hypoglycemia. On the other hand, a reduction in caudate nucleus to frontal pole connectivity was associated with a lower magnitude of favorable effects on glucose metabolism and adiposity after RYGB. These findings on the caudate are paradoxical, and might suggest that they occur as an adaptive or compensatory phenomenon, rather than as a primary mediator of metabolic improvement.
The findings may support that gastric bypass surgery partly exerts its favorable effects on energy and glucose homeostasis via the central nervous system, where changes in functional connectivity can contribute to modulation of the complex interplay between behavioral responses and neuroendocrine adaptations.
Conversely, the highlighted neural pathways may also be of relevance also for the development of obesity, insulin resistance and type 2 diabetes, and further research is warranted, including individuals in different stages of overweight and dysglycemia.
Data availability
The data and study protocol are available upon request to the corresponding author.
References
D.E. Arterburn, A.P. Courcoulas, Bariatric surgery for obesity and metabolic conditions in adults. BMJ 349, g3961 (2014).
H. Buchwald, R. Estok, K. Fahrbach, D. Banel, M.D. Jensen, W.J. Pories, J.P. Bantle, I. Sledge, Weight and Type 2 Diabetes after Bariatric Surgery: Systematic Review and Meta-analysis. Am. J. Med 122, 248 (2009).
I. Cornejo-Pareja, M. Clemente-Postigo, F.J. Tinahones, Metabolic and Endocrine Consequences of Bariatric Surgery. Front Endocrinol. (Lausanne) 10, 626 (2019).
L.E. Sewaybricker, E.A. Schur, Is Bariatric Surgery Brain Surgery? Diabetes 70, 1244 (2021).
C. Broberger, Brain regulation of food intake and appetite: Molecules and networks. J. Intern Med 258, 301 (2005).
C. Diepenbroek, M.J. Serlie, E. Fliers, A. Kalsbeek, S.E. la Fleur, Brain areas and pathways in the regulation of glucose metabolism. BioFactors 39, 505 (2013).
D. Vallöf, Glucagon-like peptide-1 and alcohol-mediated behaviors in rodents. Glucagon-like Peptide-1 and Alcohol-Mediated Behaviors in Rodents, Univeristy of Gothenburg, 2019.
J.F. Davis, D.L. Choi, S.C. Benoit, Insulin, leptin and reward. Trends Endocrinol. Metab. 21, 68 (2010).
K.P. Skibicka, C. Hansson, E. Egecioglu, S.L. Dickson, Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addiction Biol. 17, 95 (2012).
C.M. Olsen, Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology 61, 1109 (2011).
R.M. O’Connor, P.J. Kenny, Utility of ‘substance use disorder’ as a heuristic for understanding overeating and obesity. Prog. Neuropsychopharmacol. Biol. Psychiatry 118, 110580 (2022).
G.F. Koob, N.D. Volkow, Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760 (2016).
K. Coveleskie, A. Gupta, L.A. Kilpatrick, E.D. Mayer, C. Ashe-Mcnalley, J. Stains, J.S. Labus, E.A. Mayer, Altered functional connectivity within the central reward network in overweight and obese women. Nutr. Diabetes 5(1), e148 (2015).
G.J. Morton, T.H. Meek, M.W. Schwartz, Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 15, 367 (2014).
Y. Zeighami, S. Iceta, M. Dadar, M. Pelletier, M. Nadeau, L. Biertho, A. Lafortune, A. Tchernof, S. Fulton, A. Evans, D. Richard, A. Dagher, A. Michaud, Spontaneous neural activity changes after bariatric surgery: A resting-state fMRI study. Neuroimage 241, 118419 (2021).
R.J. Lepping, A.S. Bruce, A. Francisco, H.-W. Yeh, L.E. Martin, J.N. Powell, L. Hancock, T.M. Patrician, F.J. Breslin, N. Selim, J.E. Donnelly, W.M. Brooks, C.R. Savage, W.K. Simmons, J.M. Bruce, Resting-state brain connectivity after surgical and behavioral weight loss. Obesity 23, 1422 (2015).
G. Li, G. Ji, Y. Hu, M. Xu, Q. Jin, L. Liu, K.M. Deneen, J. Zhao, A. Chen, G. Cui, H. Wang, Q. Zhao, K. Wu, E. Shokri‐Kojori, D. Tomasi, N.D. Volkow, Y. Nie, Y. Zhang, G. Wang, Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self‐referential processing. Hum. Brain Mapp. 39, 4755 (2018).
G. Olivo, W. Zhou, M. Sundbom, C. Zhukovsky, P. Hogenkamp, L. Nikontovic, J. Stark, L. Wiemerslage, E.-M. Larsson, C. Benedict, H.B. Schiöth, Resting-state brain connectivity changes in obese women after Roux-en-Y gastric bypass surgery: A longitudinal study. Sci. Rep. 7, 6616 (2017).
J.J. Tuulari, H.K. Karlsson, J. Hirvonen, J.C. Hannukainen, M. Bucci, M. Helmiö, J. Ovaska, M. Soinio, P. Salminen, N. Savisto, L. Nummenmaa, P. Nuutila, Weight loss after bariatric surgery reverses insulin-induced increases in brain glucose metabolism of the morbidly obese. Diabetes 62, 2747 (2013).
K.F. Hunt, J.T. Dunn, C.W. le Roux, L.J. Reed, P.K. Marsden, A.G. Patel, S.A. Amiel, Differences in Regional Brain Responses to Food Ingestion After Roux-en-Y Gastric Bypass and the Role of Gut Peptides: A Neuroimaging Study. Diabetes Care 39, 1787 (2016).
K.E. Almby, M.H. Lundqvist, N. Abrahamsson, S. Kvernby, M. Fahlström, M.J. Pereira, M. Gingnell, F.A. Karlsson, G. Fanni, M. Sundbom, U. Wiklund, S. Haller, M. Lubberink, J. Wikström, J.W. Eriksson, Effects of Gastric Bypass Surgery on the Brain: Simultaneous Assessment of Glucose Uptake, Blood Flow, Neural Activity, and Cognitive Function During Normo- and Hypoglycemia. Diabetes 70, 1265 (2021).
S. Whitfield-Gabrieli, A. Nieto-Castanon, Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect 2, 125 (2012).
K.C. Berridge, T.E. Robinson, What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 28, 309 (1998).
M. Morales, E.B. Margolis, Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73 (2017).
R. Al-Hasani, R. Gowrishankar, G.P. Schmitz, C.E. Pedersen, D.J. Marcus, S.E. Shirley, T.E. Hobbs, A.J. Elerding, S.J. Renaud, M. Jing, Y. Li, V.A. Alvarez, J.C. Lemos, M.R. Bruchas,, Ventral tegmental area GABAergic inhibition of cholinergic interneurons in the ventral nucleus accumbens shell promotes reward reinforcement. Nat. Neurosci. 24(10), 1414 (2021).
G. Chahine, E.K. Diekhof, A. Tinnermann, O. Gruber, On the role of the anterior prefrontal cortex in cognitive “branching”: An fMRI study. Neuropsychologia 77, 421 (2015).
L.M. Yager, A.F. Garcia, A.M. Wunsch, S.M. Ferguson, The ins and outs of the striatum: Role in drug addiction. Neuroscience 301, 529 (2015).
A. de Groote and A. de Kerchove d’Exaerde, Thalamo-Nucleus Accumbens Projections in Motivated Behaviors and Addiction. Front Syst. Neurosci. 15, (2021).
B. Kim, H. Im, The role of the dorsal striatum in choice impulsivity. Ann. N. Y. Acad. Sci. 1451, 92 (2019).
S.N. Haber, Corticostriatal circuitry. Dialogues Clin. Neurosci. 18, 7 (2016).
R. Leech, D.J. Sharp, The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12 (2014).
J.A. Brewer, K.A. Garrison, S. Whitfield-Gabrieli, What about the “Self” is Processed in the Posterior Cingulate Cortex? Front Hum. Neurosci. 7, 647 (2013).
J.N. Zhu, J.J. Wang, The cerebellum in feeding control: possible function and mechanism. Cell Mol. Neurobiol. 28, 469 (2008).
A.Y.T. Low, N. Goldstein, J.R. Gaunt, K.-P. Huang, N. Zainolabidin, A.K.K. Yip, J.R.E. Carty, J.Y. Choi, A.M. Miller, H.S.T. Ho, C. Lenherr, N. Baltar, E. Azim, O.M. Sessions, T.H. Ch’ng, A.S. Bruce, L.E. Martin, M.A. Halko, R.O. Brady, L.M. Holsen, A.L. Alhadeff, A.I. Chen, J.N. Betley, Reverse-translational identification of a cerebellar satiation network. Nature 600, 269 (2021).
E.M. Marron, R. Viejo-Sobera, G. Cuatrecasas, D. Redolar-Ripoll, P.G. Lorda, A. Datta, M. Bikson, G. Magerowski, M. Alonso-Alonso, Prefronto-cerebellar neuromodulation affects appetite in obesity. Int. J. Obes. 43, 2119 (2019).
J. Cheng, J. Wang, X. Ma, R. Ullah, Y. Shen, Y.-D. Zhou, Anterior Paraventricular Thalamus to Nucleus Accumbens Projection Is Involved in Feeding Behavior in a Novel Environment. Front Mol. Neurosci. 11, 202 (2018).
G. Labouèbe, B. Boutrel, D. Tarussio, B. Thorens, Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat. Neurosci. 19(8), 999 (2016).
D.J. Christoffel, J.J. Walsh, B.D. Heifets, P. Hoerbelt, S. Neuner, G. Sun, V.K. Ravikumar, H. Wu, C.H. Halpern, R.C. Malenka, Input-specific modulation of murine nucleus accumbens differentially regulates hedonic feeding. Nat. Commun. 12(1), 1 (2021).
D. Milardi, A. Quartarone, A. Bramanti, G. Anastasi, S. Bertino, G.A. Basile, P. Buonasera, G. Pilone, G. Celeste, G. Rizzo, D. Bruschetta, A. Cacciola, The Cortico-Basal Ganglia-Cerebellar Network: Past, Present and Future Perspectives. Front Syst. Neurosci. 13, 61 (2019).
D. Ballotta, F. Talami, F. Pizza, A.E. Vaudano, F. Benuzzi, G. Plazzi, S. Meletti, Hypothalamus and amygdala functional connectivity at rest in narcolepsy type 1. Neuroimage Clin. 31, 102748 (2021).
E.E. Noble, Z. Wang, C.M. Liu, E.A. Davis, A.N. Suarez, L.M. Stein, L. Tsan, S.J. Terrill, T.M. Hsu, A.H. Jung, L.M. Raycraft, J.D. Hahn, M. Darvas, A.M. Cortella, L.A. Schier, A.W. Johnson, M.R. Hayes, D.P. Holschneider, S.E. Kanoski, Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat. Commun. 10(1), 1 (2019).
O. Contreras-Rodríguez, R. Vilar-López, Z.B. Andrews, J.F. Navas, C. Soriano-Mas, A. Verdejo-García, Altered cross-talk between the hypothalamus and non-homeostatic regions linked to obesity and difficulty to lose weight. Sci. Rep. 7(1), 1 (2017).
M.H. Lundqvist, K. Almby, N. Abrahamsson, J.W. Eriksson, Is the Brain a Key Player in Glucose Regulation and Development of Type 2 Diabetes? Front Physiol. 10, 1 (2019).
Funding
This work was supported by research grants from the Swedish Diabetes Foundation (DIA2019-490), the Exodiab - Excellence of Diabetes Research in Sweden, the Ernfors Foundation, the Swedish Society for Medical Research, the P.O. Zetterling Foundation, the Novo Nordisk Foundation (NNF20OC0063864), the European Commission via the Marie Sklodowska Curie Innovative Training Network TREATMENT (H2020-MSCA-ITN-721236), and the Uppsala University Hospital ALF grants (Swedish Government research support). Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
G.F. and J.W.E. conceptualized the study. G.F., C.K., and S.H. analyzed the data. J.W.E. supervised the work and was involved in funding acquisition. G.F. wrote the draft and E.R., M.S., S.H., and J.W.E. revised the paper and contributed with relevant intellectual content. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The study was approved by the Regional Research Ethics Committee of Uppsala (DNR 2017/210). All subjects received written and verbal information before signing an informed consent form. The study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fanni, G., Kagios, C., Roman, E. et al. Effects of gastric bypass surgery on brain connectivity responses to hypoglycemia. Endocrine 79, 304–312 (2023). https://doi.org/10.1007/s12020-022-03253-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03253-y