Abstract
Purpose
Polycystic ovary syndrome (PCOS) is a complex reproductive event that is delineated by endocrine/metabolic disorders. Alteration of kisspeptin status in the hypothalamus and adipose tissue is critical to increased endocrine/metabolic derangements in PCOS individuals, aggravating the clinical manifestation of PCOS and its complications. Short chain fatty acids (SCFAs) are crucial modulators of metabolic homeostasis. However, the role of SCFAs, particularly acetate on hypothalamic-adipose kisspeptin status (HAKS) in PCOS model is unknown. The present study hypothesized acetate as a key player in restoration of deranged HAKS, associated with experimental PCOS model.
Methods
Three groups (n = 6/group) of female Wistar rats (120–150 g) were used. The groups were treated (po) for 21 days with vehicle, letrozole (1 mg/kg) with/without acetate (200 mg/kg) respectively.
Results
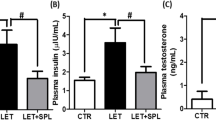
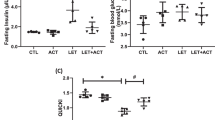
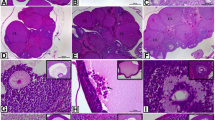
Letrozole-treated animals had impaired glucose homeostasis, elevated testosterone, leptin and LH/FSH ratio and decreased GnRH and adiponectin with ovarian tissues revealing degenerated follicles and disrupted morphology. These animals also showed increased concentration of hypothalamic triglyceride (TG)/total cholesterol (TC), free fatty acid (FFA), and decreased concentration of TG/TC/FFA in visceral adipose tissue (VAT) with an increase in hypothalamic and VAT malondialdehyde, NF-κB/TNF-α and decreased glutathione/G6PD and hypothalamic but not VAT kisspeptin. Immunohistochemical analysis revealed the expression of NLRP3 inflammasome in the hypothalamus and VAT and all these changes were attenuated by acetate.
Conclusions
Altogether, the present results demonstrate that PCOS is characterized with hypothalamic-adipose inflammation, associated with immunohistochemical expression of NLRP3 with significant alteration of hypothalamic but not adipose kisspeptin. The results suggest that acetate restores kisspeptin status in PCOS animals. This beneficial effect is accompanied by repressed NLRP3 immunoreactivity.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
A.S. Melo, R.A. Ferriani, P.A. Navarro, Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics 70, 765–769 (2015)
A.H. Balen, L.C. Morley, M. Misso, S. Franks, R.S. Legro, C.N. Wijeyaratne, E. Stener-Victorin, B.C. Fauser, R.J. Norman, H. Teede, The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 22(6), 687–708 (2016)
U. Zafar, Z. Memon, K. Moin, S. Agha, J.A. Hassan, D. Zehra, Prevalence of PCOS with associated symptoms and complications at Tertiary Care Hospital of Karachi. J. Adv. Med. Med. Res. 30(4), 1–9 (2019)
A.Z. Rutkowska, E. Diamanti-Kandarakis, Polycystic ovary syndrome and environmental toxins. Fertil. Steril. 106(4), 948–958 (2016)
Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group, Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 19(1), 41–47 (2004)
J. Liu, Q. Wu, Y. Hao, M. Jiao, X. Wang, S. Jiang, L. Han, Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum. Reprod. 36(4), 1108–19. (2021)
M. Peigné, D. Dewailly, Long term complications of polycystic ovary syndrome (PCOS). In Annales d’endocrinologie (2014) (Vol. 75, No. 4, pp. 194–199). Elsevier Masson
R. Azziz, C. Marin, L. Hoq, E. Badamgarav, P. Song, Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J. Clin. Endocrinol. Metab. 90(8), 4650–4658 (2005)
C. Meng, Nitric oxide (NO) levels in patients with polycystic ovary syndrome (PCOS): a meta-analysis. J. Int. Med. Res. 47(9), 4083–94. (2019).
W.M. Wolf, R.A. Wattick, O.N. Kinkade, M.D. Olfert, Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int. J. Environ. Res. public health 15(11), 2589 (2018)
S. Li, D. Zhu, H. Duan, Q. Tan, Genetic investigation into ethnic disparity in polycystic ovarian syndrome. Gynecol. Endocrinol. 29(10), 878–882 (2013)
S. Brakta, D. Lizneva, K. Mykhalchenko, A. Imam, W. Walker, M.P. Diamond, R. Azziz, Perspectives on polycystic ovary syndrome: is polycystic ovary syndrome research underfunded? J. Clin. Endocrinol. Metab. 102(12), 4421–4427 (2017)
E. Kandaraki, A. Chatzigeorgiou, C. Piperi, E. Palioura, S. Palimeri, P. Korkolopoulou, M. Koutsilieris, A.G. Papavassiliou, Reduced ovarian glyoxalase-I activity by dietary glycotoxins and androgen excess: a causative link to polycystic ovarian syndrome. Mol. Med. 18(8), 1183–1189 (2012)
P. Moghetti, Insulin resistance and polycystic ovary syndrome. Curr. Pharm. Des. 22(36), 5526–5534 (2016). https://doi.org/10.2174/1381612822666160720155855
E. Diamanti-Kandarakis, A.G. Papavassiliou, Outstanding questions. Trends Mol. Med. 7(12), 324–32. (2006)
F.K.H. Shirazi, Z. Khodamoradi, M. Jeddi, Insulin resistance and high molecular weight adiponectin in obese and non-obese patients with Polycystic Ovarian Syndrome (PCOS).BMC Endocr. Disord. 21, 45 (2021). :https://doi.org/10.1186/s12902-021-00710-z
L. Ibanez, E. Oberfield Sh, S.F. Witchel, R.J. Auchus, R.J. Chang, E. Codner et al. An international consortium apdate: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm. Res. Paediatr. 8896, 371–395 (2017).
N.E. Baskind, A.H. Balen, Hypothalamic–pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best. Pract. Res. Clin. Obstet. Gynaecol. 37, 80–97 (2016)
N.M. Orsi, N.E. Baskind, M. Cummings, Anatomy, development, histology, and normal function of the ovary. in Pathology of the Ovary, Fallopian Tube and Peritoneum, (Springer, London, 2014) pp. 1–32.
A.M. Moore, R.E. Campbell, The neuroendocrine genesis of polycystic ovary syndrome: a role for arcuate nucleus GABA neurons. J. Steroid Biochem. Mol. Biol. 160, 106–117 (2016)
J.E. Hall, A.E. Taylor, F.J. Hayes, W.F. Crowley, Insights into hypothalamic-pituitary dysfunction in polycystic ovary syndrome. J. Endocrinol. Investig. 21(9), 602–611 (1998).
D. Barlampa, M.S. Bompoula, A. Bargiota, S. Kalantaridou, G. Mastorakos, G. Valsamakis, Hypothalamic inflammation as a potential pathophysiologic basis for the heterogeneity of clinical, hormonal, and metabolic presentation in PCOS. Nutrients 13(2), 520 (2021)
K.S. Olaniyi, A.A. Oniyide, O.A. Adeyanju, L.S. Ojulari, A.O. Omoaghe, O.E. Olaiya, Low dose spironolactone-mediated androgen-adiponectin modulation alleviates endocrine-metabolic disturbances in letrozole-induced PCOS. Toxicol. Appl. Pharmacol. 411, 115381 (2021a)
A.P. Delitala, G. Capobianco, G. Delitala, P.L. Cherchi, S. Dessole, Polycystic ovary syndrome, adipose tissue and metabolic syndrome. Arch. Gynecol. Obstet. 296(3), 405–419 (2017)
P.M. Spritzer, S.B. Lecke, F. Satler, D.M. Morsch, Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction 149(5), R219–R227 (2015)
R. Pasquali, Lifestyle Interventions and Natural and Assisted Reproduction in Patients with PCOS. InInfertility in Women with Polycystic Ovary Syndrome 2018 (pp. 169–180). Springer, Cham
B. Zeng, Z. Lai, L. Sun, Z. Zhang, J. Yang, Z. Li, J. Lin, Z. Zhang, Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res. Microbiol. 170(1), 43–52 (2019)
L.G. Silveira, C. Tusset, A.C. Latronico, Impact of mutations in kisspeptin and neurokinin B signaling pathways on human reproduction. Brain Res. 1364, 72–80 (2010)
B.S. Araújo, M.C. Baracat, R. dos Santos Simões, C. de Oliveira Nuñes, G.A. Maciel, R.A. Lobo, J.M. Soares-Jr, E.C. Baracat, Kisspeptin Influence on Polycystic Ovary Syndrome—A Mini Review. Reprod. Sci. 27(2), 455–460 (2020)
R.E. Brown, D.A. Wilkinson, S.A. Imran, A. Caraty, M. Wilkinson, Hypothalamic kiss1 mRNA and kisspeptin immunoreactivity are reduced in a rat model of polycystic ovary syndrome (PCOS). Brain Res. 1467, 1–9 (2012).
S. Osuka, A. Iwase, T. Nakahara, M. Kondo, A. Saito, T. Nakamura, S. Takikawa, M. Goto, T. Kotani, F. Kikkawa, Kisspeptin in the hypothalamus of 2 rat models of polycystic ovary syndrome. Endocrinology 158(2), 367–377 (2017).
J.C. Venancio, L.O. Margatho, R. Rorato, R.R. Rosales, L.K. Debarba, R. Coletti, J. Antunes-Rodrigues, C.F. Elias, L.L. Elias, Short-term high-fat diet increases leptin activation of CART neurons and advances puberty in female mice. Endocrinology 158(11), 3929–3942 (2017)
C.Y. Lee, S. Li, X.F. Li, D.A. Stalker, C. Cooke, B. Shao, H. Kelestimur, B.A. Henry, G. Conductier, K.T. O’Byrne, I.J. Clarke, Lipopolysaccharide reduces gonadotrophin-releasing hormone (GnRH) gene expression: Role of RFamide-related peptide-3 and kisspeptin. Reprod., Fertil. Dev. 31(6), 1134–1143 (2019)
M.A. González Hernández, E.E. Canfora, J.W. Jocken, E.E. Blaak, The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 11(8), 1943 (2019)
K.S. Olaniyi, O.A. Amusa, Sodium acetate-mediated inhibition of histone deacetylase alleviates hepatic lipid dysregulation and its accompanied injury in streptozotocin-nicotinamide-induced diabetic rats. Biomedicine Pharmacother. 128, 110226 (2020)
V. Karoor, D. Strassheim, T. Sullivan, A. Verin, N.S. Umapathy, E.C. Dempsey, D.N. Frank, K.R. Stenmark, E. Gerasimovskaya, The short-chain fatty acid butyrate attenuates pulmonary vascular remodeling and inflammation in hypoxia-induced pulmonary hypertension. Int. J. Mol. Sci. 22(18), 9916 (2021)
O.A. Adeyanju, T.O. Falodun, O.S. Michael, O.A. Soetan, A.L. Oyewole, R.D. Agbana, Spironolactone reversed hepato-ovarian triglyceride accumulation caused by letrozole-induced polycystic ovarian syndrome: tissue uric acid—a familiar foe. Naunyn-Schmiedeberg’s Arch. Pharmacol. 393(6), 1055–1066 (2020).
H. Kafali, M. Iriadam, I. Ozardali, N. Demir, Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch. Med. Res. 35, 103–108 (2004)
K.S. Olaniyi, M.N. Owolabi, C.L. Atuma, T.B. Agunbiade, B.Y. Alabi, Acetate rescues defective brain-adipose metabolic network in obese Wistar rats by modulation of peroxisome proliferator-activated receptor-γ. Sci. Rep. 11(1), 1–5 (2021b)
A.W. Hsing, Y.T. Gao, S. Chua Jr, J. Deng, F.Z. Stanczyk, Insulin resistance and prostate cancer risk. J. Natl Cancer Inst. 95(1), 67–71 (2003)
K.S. Olaniyi, L.A. Olatunji, Inhibition of pyruvate dehydrogenase kinase-4 by l-glutamine protects pregnant rats against fructose-induced obesity and hepatic lipid accumulation. Biomed. Pharmacother. 110, 59–67 (2019)
S. Malamed, J.A. Gibney, S.R. Ojeda, Ovarian innervation develops before initiation of folliculogenesis in the rat. Cell Tissue Res. 270(1), 87–93 (1992)
K.S. Olaniyi, O.A. Amusa, I.O. Ajadi, B.Y. Alabi, T.B. Agunbiade, M.B. Ajadi, Repression of HDAC5 by acetate restores hypothalamic-pituitary-ovarian function in type 2 diabetes mellitus. Reprod. Toxicol. 106, 69–81 (2021)
K.A. Posey, D.J. Clegg, R.L. Printz, J. Byun, G.J. Morton, A. Vivekanandan-Giri, S. Pennathur, D.G. Baskin, J.W. Heinecke, S.C. Woods, M.W. Schwartz, Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol.-Endocrinol. Metab. 296(5), E1003–E1012 (2009)
I. Kojta, M. Chacińska, A.Błachnio-Zabielska,, Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients 12(5), 1305 (2020). https://doi.org/10.3390/nu12051305
K.S. Olaniyi, L.A. Olatunji, L-glutamine ameliorates adipose-hepatic dysmetabolism in OC-treated female rats. J. Endocrinol. 246(1), 1–12 (2020). https://doi.org/10.1530/JOE-19-0582
S. Cinti, Adipose organ development and remodeling. Compr. Physiol. 8(4), 1357–1431 (2018). https://doi.org/10.1002/cphy.c170042
K. Sun, C.M. Kusminski, P.E. Scherer, Adipose tissue remodeling and obesity. J. Clin. Investig. 121(6), 2094–2101 (2011). https://doi.org/10.1172/JCI45887
A.T. Vieira, I. Galvão, L.M. Macia, E.M. Sernaglia, M.A. Vinolo, C.C. Garcia, L.P. Tavares, F.A. Amaral, L.P. Sousa, F.S. Martins, C.R. Mackay, Dietary fiber and the short‐chain fatty acid acetate promote resolution of neutrophilic inflammation in a model of gout in mice. J. Leukoc. Biol. 101(1), 275–84. (2017)
M.A. Vinolo, H.G. Rodrigues, R.T. Nachbar, R. Curi, Regulation of inflammation by short chain fatty acids. Nutrients 3(10), 858–876 (2011)
Author information
Authors and Affiliations
Contributions
Conceptualization and design of this research were attributed to K.S.O. The experiment, analysis and interpretation of data were performed by K.S.O. and S.E.A., and K.S.O. and M.B.O. contributed reagents to the study. K.S.O. and S.E.A. drafted the manuscript. K.S.O., S.E.A., and M.B.O. read, revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olaniyi, K.S., Areloegbe, S.E. & Oyeleke, M.B. Acetate restores hypothalamic-adipose kisspeptin status in a rat model of PCOS by suppression of NLRP3 immunoreactivity. Endocrine 78, 628–640 (2022). https://doi.org/10.1007/s12020-022-03191-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03191-9