Abstract
Purpose
We aimed to illustrate gut microbiota and short chain fatty acid (SCFA) levels in diabetic nephropathy (DN) patients, and investigate the mechanism of sodium butyrate in diabetic mellitus (DM) rats.
Methods
Gut microbiota and serum SCFA levels were measured by 16S rDNA and GC-MS. After being built by streptozotocin (DM rats), the DM rats were administered 300 mg/kg sodium butyrate for 12 weeks (DM + BU rats). Gut microbiota, serum and fecal butyrate level were measured. RT-PCR, WB and transmission electron microscopy were performed to explore LC3mRNA or LC3B protein expression, and autophagosomes in kidney tissues. AMPK/mTOR protein expression in renal tissue were also measured.
Results
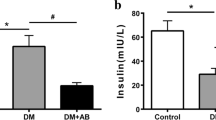
The gut microbial dysbiosis was found in DM and DN groups, and some SCFAs-producing bacteria were decreased in DN group. The serum butyrate concentrations were lower in SCFA-DN group compared with SCFA-HC group and SCFA-DM group in the other cohort. Serum butyrate level was positively correlated with eGFR. Sodium butyrate increased serum and fecal butyrate levels, and improved the enlargement of glomerular area and fibronectin and collagen IV expressions in renal tissues in DM + BU rats. The LC3 mRNA, LC3BII/I ratio and number of autophagosomes were increased in renal tissue of DM + BU rats. Higher p-AMPK/AMPK ratio and lower p-mTOR/ mTOR ratio were shown in renal tissue of DM + BU rats compared with DM rats.
Conclusions
We found the decrease in SCFAs-producing bacteria and low SCFAs concentrations in DN patients. Oral butyrate supplementation may improve kidney injury in DM rats, possibly by increasing autophagy via activating AMPK/mTOR pathway.





Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request
References
Y.Z. Li, T. Di, X.G. Shi et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 369, m997 (2020)
R.T. Demmer, A.M. Zuk, M. Rosenbaum, M. Desvarieux, Prevalence of diagnosed and undiagnosed type 2 diabetes mellitus among us adolescents: results from the continuous NHANES, 1999–2010. Am J Epidemiol 178(7), 1106–1113 (2013)
R.Z. Alicic, E.J. Johnson, K.R. Tuttle, SGLT2 Inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kidney Dis 72(2), 267–277 (2018)
R.Z. Alicic, M.T. Rooney, K.R. Tuttle, Diabetic kidney disease challenges, progress, and possibilities. Clin J Am Soc Nephrol 12(12), 2032–2045 (2017)
H.J. Anders, K. Andersen, B. Stecher, The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 83(6), 1010–1016 (2013)
J.J. Qin, Y.R. Li, Z.M. Cai et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 490(7418), 55–60 (2012)
T. Høverstad, T. Midtvedt, Short-chain fatty acids in germfree Mice and Rats1. J Nutr 116(9), 1772–1776 (1986)
S.Q. Wang, D. Lv, S.H. Jiang et al. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin Sci (Lond) 133(17), 1857–1870 (2019)
A. Jacobson, L. Lam, M. Rajendram et al. A gut commensal-produced metabolite mediates colonization resistance to salmonella infection. Cell Host Microbe 24(2), 296–307 (2018)
V. Andrade-Oliveira, M.T. Amano, M. Correa-Costa et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol 26(8), 1877–1888 (2015)
N. Liu, L.Q. Xu, Y.F. Shi, S.G. Zhuang, Podocyte autophagy: a potential therapeutic target to prevent the progression of diabetic nephropathy. J Diabetes Res 2017, 3560238 (2017)
S. Alers, A.S. Löffler, S. Wesselborg, B. Stork, Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32(1), 2–11 (2011)
W. Huang, Y. Man, C. Gao et al. Short-chain fatty acids ameliorate diabetic nephropathy via GPR43-mediated inhibition of oxidative stress and NF-κB signaling. Oxid Med Cell Longev 2020, 4074832 (2020)
P.H. Marathe, H.X. Gao, K.L. Close, American diabetes association standards of medical care in Diabetes 2017. J Diabetes. 9(4), 320–324 (2017)
T.K. Hansen, L. Tarnow, S. Thiel et al. Association between mannose-binding lectin and vascular complications in type 1 diabetes. Diabetes. 53(6), 1570–1576 (2004)
T.W. Tervaert, A.L. Mooyaart, K. Amann et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21(4), 556–563 (2010)
Y. Sun, C. Zhou, Y. Chen, X. He, F. Gao, D. Xue, Quantitative increase in short-chain fatty acids, especially butyrate protects kidney from ischemia/reperfusion injury. J Investig Med 2020, 001715 (2021)
N. Mizushima, T. Yoshimori, B. Levine, Methods in mammalian autophagy research. Cell. 140(3), 313–326 (2010)
M. Hamasaki, N. Furuta, A. Matsuda et al. Autophagosomes form at ER–mitochondria contact sites. Nature. 495(7441), 389–393 (2013)
M.Y. Kim, J.H. Lim, H.H. Youn et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK–SIRT1–PGC1α axis in db/db mice. Diabetologia 56(1), 204–217 (2013)
S.B. Tao, L.Z. Li, L. Li et al. Understanding the gut–kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: an analysis of the gut microbiota composition. Acta Diabetol 56(5), 581–592 (2019)
Z.G. Ren, A. Li, J.W. Jiang et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 68(6), 1014–1023 (2019)
Y. Yamaguchi, K. Adachi, T. Sugiyama et al. Association of intestinal microbiota with metabolic markers and dietary habits in patients with type 2 diabetes. Digestion. 94(2), 66–72 (2016)
Q. Feng, S.S. Liang, H.J. Jia et al. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat Commun 6, 6528 (2015)
E.B. Daliri, C.N. Tango, B.H. Lee, D.H. Oh, Human microbiome restoration and safety. Int J Med Microbiol 308(5), 487–497 (2018)
W.P. Dong, Y. Jia, X.X. Liu et al. Sodium butyrate activates NRF2 to ameliorate diabetic nephropathy possibly via inhibition of HDAC. J Endocrinol 232(1), 71–83 (2017)
Y. Du, G. Tang, W.J. Yuan, Suppression of HDAC2 by sodium butyrate alleviates apoptosis of kidney cells in db/db mice and HGinduced NRK52E cells. Int J Mol Med 45(1), 210–222 (2019)
Y.J. Li, X.C. Chen, T.K. Kwan et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid–mediated activation of G protein–coupled receptors GPR43 and GPR109A. J Am Soc Nephrol 31(6), 1267–1281 (2020)
Y. Li, X.H. Su, Y. Gao et al. The potential role of the gut microbiota in modulating renal function in experimental diabetic nephropathy murine models established in same environment. Biochim Biophys Acta Mol Basis Dis 1866(6), 165764 (2020)
M. Kobayashi, D. Mikami, H. Kimura et al. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun 486(2), 499–505 (2017)
J.L. Gu, W. Huang, W.Q. Zhang et al. Sodium butyrate alleviates high-glucose-induced renal glomerular endothelial cells damage via inhibiting pyroptosis. Int Immunopharmacol 75, 105832 (2019)
A.D.A. Barbosa Júnior, H. Zhou, D. HÜltenschmidt, V. Totovic, N. Jurilj, U. Pfeife, Inhibition of cellular autophagy in proximal tubular cells of the kidney in streptozotocin-diabetic and uninephrectomized rats. Virchows Arch B Cell Pathol Incl Mol Patho 61(6), 359–366 (1992)
C.M. Qiao, M.F. Sun, X.B. Jia et al. Sodium butyrate causes α-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp Cell Res 387(1), 111772 (2020)
J.T. Zhang, M. Yi, L.Y. Zha et al. Sodium butyrate induces endoplasmic reticulum stress and autophagy in colorectal cells: implications for apoptosis. PLoS One 11(1), e0147218 (2016)
S.L. Luo, Z.Y. Li, L.Z. Mao, S.Q. Chen, S.X. Sun, Sodium butyrate induces autophagy in colorectal cancer cells through LKB1/AMPK signaling. J Physiol Biochem 75(1), 53–63 (2018)
A. Gonzalez, R. Krieg, H. Massey et al. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol Dial Transplant 34(5), 783–794 (2019)
F.F. Wang, H.S. Wu, M.J. Fan et al. Sodium butyrate inhibits migration and induces AMPK‐mTOR pathway‐dependent autophagy and ROS‐mediated apoptosis via the miR‐139‐5p/Bmi‐1 axis in human bladder cancer cells. FASEB J 34(3), 4266–4282 (2020)
F. Gao, Y.W. Lv, J. Long et al. Butyrate improves the metabolic disorder and gut microbiome dysbiosis in mice induced by a high-fat diet. Front Pharmacol 10, 1040 (2019)
Si X., Shang W.T., Zhou Z.K., et al. Gut microbiome-induced shift of acetate to butyrate positively manages dysbiosis in high fat diet. Mol Nutr Food Res. 62(3), (2018). https://doi.org/10.1002/mnfr.201700670
N. Roshanravan, R. Mahdavi, E. Alizadeh et al. Effect of butyrate and inulin supplementation on glycemic status, lipid profile and glucagon-like peptide 1 level in patients with type 2 diabetes: a randomized double-blind, placebo-controlled trial. Horm Metab Res 49(11), 886–891 (2017)
L.L. Jia, D.Y. Li, N.H. Feng et al. Anti-diabetic effects of clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in type 2 diabetic mice. Sci Rep 7(1), 7046 (2017)
H. Yadav, J.H. Lee, J. Lloyd, P. Walter, S.G. Rane, Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem 288(35), 25088–25097 (2013)
T. Jin, J.P. Weng, Hepatic functions of GLP-1 and its based drugs: current disputes and perspectives. Am J Physiol Endocrinol Metab 311(3), 620–627 (2016)
V.A. Lizunov, J.P. Lee, M.C. Skarulis, J. Zimmerberg, S.W. Cushman, K.G. Stenkula, Impaired tethering and fusion of GLUT4 vesicles in insulin-resistant human adipose cells. Diabetes. 62(9), 3114–3119 (2013)
I.P. Salt, D.G. Hardie, AMP-activated protein kinase: an ubiquitous signaling pathway with key roles in the cardiovascular system. Circ Res 120(11), 1825–1841 (2017)
Acknowledgements
We acknowledge Qili Shi, studying in Fudan University for his expert technical assistance
Author contribution
Research idea and study design: F.H., K.D.C. and Y.H.M.; sample collection: K.C. Y.H.M., C.Y.Z. and P.P.R.; data acquisition and analysis: F.H., K.D.C., Y.H.M.; Experiments performed: F.H., K.D.C., Y.H.M., F.H.C. X.H.H. and L.X.; supervision or mentorship: F.H., Q.L., J.H.C. F.H. and K.D.C. wrote the first draft of the manuscript. F.H. reviewed and edited the manuscript. K.D.C. and Y.H.M. contributed equally in the study. All the authors approved of the final version of the manuscript.
Funding
This study was supported by the funds from National Key R&D Program of China (2018YFC1314003), Zhejiang Provincial Natural Science Foundation of China (LY20H05005, Q19H050030), Medical Scientific Research Foundation of Zhejiang Province, China (2019KY174) and Ningbo Public Service Technology Foundation, China (2019C50084).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The research protocols were conformed to the provisions of the Declaration of Helsinki and were approved by the Ethic Committee of the First Affiliated Hospital of Zhejiang University School of Medicine and HwaMei Hospital, University of Chinese Academy of Sciences.
Consent to participate
Written informed consents were obtained from the patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Cai, K., Ma, Y., Cai, F. et al. Changes of gut microbiota in diabetic nephropathy and its effect on the progression of kidney injury. Endocrine 76, 294–303 (2022). https://doi.org/10.1007/s12020-022-03002-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03002-1