Abstract
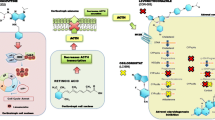
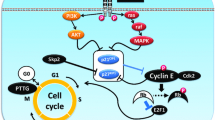
Cushing’s disease (CD), caused by an adrenocorticotropin-secreting pituitary adenoma, leads to hypercortisolemia and causes serious morbidity and increased mortality when suboptimally treated. Currently, the genetic events have rarely been reported in this disease. Recently, the recurrent activating mutations in the gene encoding ubiquitin-specific protease 8 (USP8) in CD have been independently reported by two teams. These hotspot mutations sustain epidermal growth factor receptor (EGFR) signaling and expand the pathogenic role of USP8 in corticotroph adenoma. This review summarizes current knowledge of USP8 and its substrate EGFR in cancer therapy and possible application of them in CD.
Similar content being viewed by others
References
J. Newell-Price et al., Cushing’s syndrome. Lancet 367(9522), 1605–1617 (2006)
B.M. Biller et al., Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J. Clin. Endocrinol. Metab. 93(7), 2454–2462 (2008)
A.B. Atkinson et al., Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clin. Endocrinol. 63(5), 549–559 (2005)
C.G. Patil et al., Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J. Clin. Endocrinol. Metab. 93(2), 358–362 (2008)
F. Castinetti et al., Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J. Clin. Endocrinol. Metab. 94(9), 3400–3407 (2009)
J. Jagannathan et al., Gamma Knife surgery for Cushing’s disease. J. Neurosurg. 106(6), 980–987 (2007)
C. de Bruin et al., Coexpression of dopamine and somatostatin receptor subtypes in corticotroph adenomas. J. Clin. Endocrinol. Metab. 94(4), 1118–1124 (2009)
D.L. Batista et al., The effects of SOM230 on cell proliferation and adrenocorticotropin secretion in human corticotroph pituitary adenomas. J. Clin. Endocrinol. Metab. 91(11), 4482–4488 (2006)
M. Boscaro et al., Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J. Clin. Endocrinol. Metab. 94(1), 115–122 (2009)
R. Pivonello et al., Pasireotide treatment significantly improves clinical signs and symptoms in patients with Cushing’s disease: results from a Phase III study. Clin. Endocrinol. 81(3), 408–417 (2014)
A. Godbout et al., Cabergoline monotherapy in the long-term treatment of Cushing’s disease. Eur. J. Endocrinol. 163(5), 709–716 (2010)
R. Pivonello et al., The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J. Clin. Endocrinol. Metab. 94(1), 223–230 (2009)
R. De Vecchis, C. Esposito, C. Ariano, Cabergoline use and risk of fibrosis and insufficiency of cardiac valves. Meta-analysis of observational studies. Herz 38(8), 868–880 (2013)
R.A. Feelders et al., Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N. Engl. J. Med. 362(19), 1846–1848 (2010)
F. Castinetti et al., Ketoconazole in Cushing’s disease: is it worth a try? J. Clin. Endocrinol. Metab. 99(5), 1623–1630 (2014)
M. Fleseriu et al., Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J. Clin. Endocrinol. Metab. 97(6), 2039–2049 (2012)
D. Dworakowska, A.B. Grossman, The molecular pathogenesis of corticotroph tumours. Eur. J. Clin. Invest. 42(6), 665–676 (2012)
M. Reincke et al., Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 47(1), 31–38 (2015)
L.G. Perez-Rivas et al., The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J. Clin. Endocrinol. Metab. 100(7), E997–E1004 (2015)
Z.Y. Ma et al., Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 25(3), 306–317 (2015)
E. Mizuno, N. Kitamura, M. Komada, 14-3-3-dependent inhibition of the deubiquitinating activity of UBPY and its cancellation in the M phase. Exp. Cell Res. 313(16), 3624–3634 (2007)
I.M. Meijer et al., The Usp8 deubiquitination enzyme is post-translationally modified by tyrosine and serine phosphorylation. Cell Signal 25(4), 919–930 (2013)
S. Naviglio et al., UBPY: a growth-regulated human ubiquitin isopeptidase. EMBO J. 17(12), 3241–3250 (1998)
D. Popovic, D. Vucic, I. Dikic, Ubiquitination in disease pathogenesis and treatment. Nat. Med. 20(11), 1242–1253 (2014)
H. Tanno, M. Komada, The ubiquitin code and its decoding machinery in the endocytic pathway. J. Biochem. 153(6), 497–504 (2013)
A. Ciechanover, The unravelling of the ubiquitin system. Nat. Rev. Mol. Cell Biol. 16(5), 322–324 (2015)
S.M. Nijman et al., A genomic and functional inventory of deubiquitinating enzymes. Cell 123(5), 773–786 (2005)
S. Hussain, Y. Zhang, P.J. Galardy, DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle 8(11), 1688–1697 (2009)
B. Nicholson et al., Deubiquitinating enzymes as novel anticancer targets. Future Oncol. 3(2), 191–199 (2007)
N. Saini, A. Mahindra, Therapeutic strategies for the treatment of multiple myeloma. Discov. Med. 15(83), 251–258 (2013)
E. Mizuno et al., Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol. Biol. Cell 16(11), 5163–5174 (2005)
J.B. Johnston et al., Targeting the EGFR pathway for cancer therapy. Curr. Med. Chem. 13(29), 3483–3492 (2006)
Z. Zhang et al., EGFR-mutated lung cancer: a paradigm of molecular oncology. Oncotarget 1(7), 497–514 (2010)
F. Ciardiello, G. Tortora, EGFR antagonists in cancer treatment. N. Engl. J. Med. 358(11), 1160–1174 (2008)
A.J. Mantha et al., Targeting the mevalonate pathway inhibits the function of the epidermal growth factor receptor. Clin. Cancer Res. 11(6), 2398–2407 (2005)
E. Kumaraswamy et al., BRCA1 regulation of epidermal growth factor receptor (EGFR) expression in human breast cancer cells involves microRNA-146a and is critical for its tumor suppressor function. Oncogene (2014). doi:10.1038/onc.2014.363
M. Theodoropoulou et al., Expression of epidermal growth factor receptor in neoplastic pituitary cells: evidence for a role in corticotropinoma cells. J. Endocrinol. 183(2), 385–394 (2004)
V.K. LeRiche, S.L. Asa, S. Ezzat, Epidermal growth factor and its receptor (EGF-R) in human pituitary adenomas: EGF-R correlates with tumor aggressiveness. J. Clin. Endocrinol. Metab. 81(2), 656–662 (1996)
G. Kontogeorgos et al., Localization of epidermal growth factor (EGF) and epidermal growth factor receptor (EGFr) in human pituitary adenomas and nontumorous pituitaries: an immunocytochemical study. Endocr. Pathol. 7(1), 63–70 (1996)
H. Fukuoka et al., EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J. Clin. Invest. 121(12), 4712–4721 (2011)
E. Lengyel et al., C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int. J. Cancer 113(4), 678–682 (2005)
I. Canadas et al., C-MET as a new therapeutic target for the development of novel anticancer drugs. Clin. Transl. Oncol. 12(4), 253–260 (2010)
A.T. De Oliveira et al., MET Is highly expressed in advanced stages of colorectal cancer and indicates worse prognosis and mortality. Anticancer Res. 29(11), 4807–4811 (2009)
S. Dhillon, Gefitinib: a review of its use in adults with advanced non-small cell lung cancer. Target Oncol. 10(1), 153–170 (2015)
A. Tamiya et al., Phase II trial of carboplatin, S-1, and gefitinib as first-line triplet chemotherapy for advanced non-small cell lung cancer patients with activating epidermal growth factor receptor mutations. Med. Oncol. 32(3), 40 (2015)
N. Singh, A. Jindal, D. Behera, Erlotinib usage after prior treatment with gefitinib in advanced non-small cell lung cancer: A clinical perspective and review of published literature. World J. Clin. Oncol. 5(5), 858–864 (2014)
D.R. Camidge et al., Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 13(10), 1011–1019 (2012)
S. Byun et al., USP8 is a novel target for overcoming gefitinib resistance in lung cancer. Clin. Cancer Res. 19(14), 3894–3904 (2013)
A. Colao, S. Savastano, Medical treatment of prolactinomas. Nat. Rev. Endocrinol. 7(5), 267–278 (2011)
Acknowledgments
This work was supported by Grants to Qingfang Sun from National Natural Science Foundation of China (No: 81270856).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Jian, F., Cao, Y., Bian, L. et al. USP8: a novel therapeutic target for Cushing’s disease. Endocrine 50, 292–296 (2015). https://doi.org/10.1007/s12020-015-0682-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0682-y