Abstract
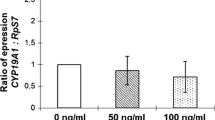
Kit ligand (KITL) is an important granulosa cell-derived growth factor in ovarian folliculogenesis, but its expression and function in human granulosa cells are currently poorly understood. Based on studies performed in animal models, it was hypothesised that KITL gene expression in human granulosa cells is regulated by androgens and/or growth differentiation factor 9 (GDF9). We utilised two models of human granulosa cells, the KGN granulosa tumour cell line and cumulus granulosa cells obtained from preovulatory follicles of women undergoing assisted reproduction. Cells were treated with combinations of 5α-dihydrotestosterone (DHT), recombinant mouse GDF9, and the ALK4/5/7 inhibitor SB431542. KITL mRNA levels were measured by quantitative real-time PCR. No change in KITL mRNA expression was observed after DHT treatment under any experimental conditions, but GDF9 treatment resulted in a significant decrease in KITL mRNA levels in both KGN and cumulus cells. The effect of GDF9 was abolished by the addition of SB431542. These results indicate that KITL is not directly regulated by androgen signalling in human granulosa cells. Moreover, this study provides the first evidence that GDF9 negatively regulates KITL gene expression in human granulosa cells providing new information on the regulation of these important growth factors in the human ovary.





Similar content being viewed by others
References
K. Manova et al., Gonadal expression of c-kit encoded at the W locus of the mouse. Development 110(4), 1057–1069 (1990)
J.A. Parrott, M.K. Skinner, Direct actions of kit-ligand on theca cell growth and differentiation during follicle development. Endocrinology 138(9), 3819–3827 (1997)
E.J. Huang et al., Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol. Biol. Cell 3(3), 349–362 (1992)
M.A. Bedell et al., DNA rearrangements located over 100 kb 5′ of the steel (Sl)-coding region in steel-panda and steel-contrasted mice deregulate Sl expression and cause female sterility by disrupting ovarian follicle development. Genes Dev. 9(4), 455–470 (1995)
S.M. Coutts et al., Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Dev. Biol. 314(1), 189–199 (2008)
J.A. Elvin et al., Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol. Endocrinol. 13(6), 1035–1048 (1999)
J.A. Elvin et al., Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol. Endocrinol. 13(6), 1018–1034 (1999)
I.M. Joyce et al., Comparison of recombinant growth differentiation factor-9 and oocyte regulation of KIT ligand messenger ribonucleic acid expression in mouse ovarian follicles. Biol. Reprod. 63(6), 1669–1675 (2000)
I.M. Joyce et al., Oocyte regulation of kit ligand expression in mouse ovarian follicles. Dev. Biol. 214(2), 342–353 (1999)
G.M. Kidder, B.C. Vanderhyden, Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 88(4), 399–413 (2010)
F. Otsuka, S. Shimasaki, A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc. Natl. Acad. Sci. USA 99(12), 8060–8065 (2002)
F.H. Thomas et al., Follicle-stimulating hormone regulates oocyte growth by modulation of expression of oocyte and granulosa cell factors. Endocrinology 146(2), 941–949 (2005)
F.H. Thomas et al., Kit ligand 2 promotes murine oocyte growth in vitro. Biol. Reprod. 78(1), 167–175 (2008)
F.H. Thomas, B.C. Vanderhyden, Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod. Biol. Endocrinol. 4, 19 (2006)
H. Yoshida et al., Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev. Biol. 184(1), 122–137 (1997)
U.A. Vitt et al., Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol. Reprod. 67(2), 473–480 (2002)
N. Kaivo-Oja et al., Adenoviral gene transfer allows Smad-responsive gene promoter analyses and delineation of type I receptor usage of transforming growth factor-beta family ligands in cultured human granulosa luteal cells. J. Clin. Endocrinol. Metab. 90(1), 271–278 (2005)
S. Mazerbourg et al., Growth differentiation factor-9 signalling is mediated by the type I receptor, activin receptor-like kinase 5. Mol. Endocrinol. 18(3), 653–665 (2004)
Q. Li et al., Transforming growth factor beta receptor type 1 is essential for female reproductive tract integrity and function. PLoS Genet. 7(10), e1002320 (2011)
D.G. Mottershead et al., Growth differentiation factor 9: bone morphogenetic protein 15 (GDF9: BMP15) synergism and protein heterodimerization. Proc. Natl. Acad. Sci. USA 110(25), E2257 (2013)
J. Peng et al., Growth differentiation factor 9: bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc. Natl. Acad. Sci. USA 110(8), E776–E785 (2013)
N. Kaivo-Oja et al., Growth differentiation factor-9 induces Smad2 activation and inhibin B production in cultured human granulosa-luteal cells. J. Clin. Endocrinol. Metab. 88(2), 755–762 (2003)
H. Shiina et al., Premature ovarian failure in androgen receptor-deficient mice. Proc. Natl. Acad. Sci. USA 103(1), 224–229 (2006)
A. Dai et al., Orphan nuclear receptor nur77 regulates androgen receptor gene expression in mouse ovary. PLoS One 7(6), e39950 (2012)
Y. Nishi et al., Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 142(1), 437–445 (2001)
M.L. Grondahl et al., Specific genes are selectively expressed between cumulus and granulosa cells from individual human pre-ovulatory follicles. Mol. Hum. Reprod. 18(12), 572–584 (2012)
S. Koks et al., The differential transcriptome and ontology profiles of floating and cumulus granulosa cells in stimulated human antral follicles. Mol. Hum. Reprod. 16(4), 229–240 (2010)
S. Varani et al., Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol. Endocrinol. 16(6), 1154–1167 (2002)
D.G. Mottershead et al., Characterization of recombinant human growth differentiation factor-9 signalling in ovarian granulosa cells. Mol. Cell. Endocrinol. 283(1–2), 58–67 (2008)
C.M. Simpson et al., Activation of latent human GDF9 by a single residue change (Gly 391 Arg) in the mature domain. Endocrinology 153(3), 1301–1310 (2012)
D.G. Mottershead, L.J. Ritter, R.B. Gilchrist, Signalling pathways mediating specific synergistic interactions between GDF9 and BMP15. Mol. Hum. Reprod. 18(3), 121–128 (2012)
D.D. Fr, R. Tarlatzis, Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 81(1), 19–25 (2004)
D.D. Fr, R. Tarlatzis, Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 19(1), 41–47 (2004)
G.J. Inman et al., SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62(1), 65–74 (2002)
M.M. Pulkki et al., A covalently dimerized recombinant human bone morphogenetic protein-15 variant identifies bone morphogenetic protein receptor type 1B as a key cell surface receptor on ovarian granulosa cells. Endocrinology 153(3), 1509–1518 (2012)
S.A. McGrath, A.F. Esquela, S.J. Lee, Oocyte-specific expression of growth/differentiation factor-9. Mol. Endocrinol. 9(1), 131–136 (1995)
M. Nomura et al., Activin stimulates CYP19A gene expression in human ovarian granulosa cell-like KGN cells via the Smad2 signaling pathway. Biochem. Biophys. Res. Commun. 436(3), 443–448 (2013)
M.S. Butler et al., Small glutamine-rich tetratricopeptide repeat-containing protein alpha is present in human ovaries but may not be differentially expressed in relation to polycystic ovary syndrome. Fertil. Steril. 99(7), 2076–2083.e1 (2013)
S. Chadha et al., Androgen receptor expression in human ovarian and uterine tissue of long-term androgen-treated transsexual women. Hum. Pathol. 25(11), 1198–1204 (1994)
H.J. Ahn et al., Parabens inhibit the early phase of folliculogenesis and steroidogenesis in the ovaries of neonatal rats. Mol. Reprod. Dev. 79(9), 626–636 (2012)
D. Huansheng et al., Estrogen inhibits the early development of mouse follicles through regulating the expression of Kit ligand. Biochem. Biophys. Res. Commun. 410(3), 659–664 (2011)
S. Weil et al., Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J. Clin. Endocrinol. Metab. 84(8), 2951–2956 (1999)
B. Planz et al., Androgen responsiveness of stromal cells of the human prostate: regulation of cell proliferation and keratinocyte growth factor by androgen. J. Urol. 160(5), 1850–1855 (1998)
T.E. Hickey et al., Androgens augment the mitogenic effects of oocyte-secreted factors and growth differentiation factor 9 on porcine granulosa cells. Biol. Reprod. 73(4), 825–832 (2005)
J.L. Yang et al., Testosterone induces redistribution of forkhead box-3a and down-regulation of growth and differentiation factor 9 messenger ribonucleic acid expression at early stage of mouse folliculogenesis. Endocrinology 151(2), 774–782 (2010)
F.L. Teixeira Filho et al., Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 87(3), 1337–1344 (2002)
L.N. Wei et al., Abnormal expression of growth differentiation factor 9 and bone morphogenetic protein 15 in stimulated oocytes during maturation from women with polycystic ovary syndrome. Fertil. Steril. 96(2), 464–468 (2011)
K. Adekola, M. Agulnik, Advances in adjuvant therapy of gastrointestinal stromal tumors. Curr. Oncol. Rep. 14(4), 327–332 (2012)
C.L. Corless, C.M. Barnett, M.C. Heinrich, Gastrointestinal stromal tumours: origin and molecular oncology. Nat. Rev. Cancer 11(12), 865–878 (2011)
R.P. Dematteo et al., Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 373(9669), 1097–1104 (2009)
Acknowledgments
We thank Dr Hamish Hamilton, Dr Marcin Stankiewicz, the nurses and embryology staff, and consenting patients at Flinders Reproductive Medicine for reviewing patient information, assistance in consenting patients and collecting tissues, or donating tissue for the purposes of this study. We acknowledge Professors Hajime Nawata and Toshihiko Yanase of Kyushu University and Professor Yoshihiro Nishi of Kurume University for creating and allowing us to use the KGN cell line. This work was supported by the National Health and Medical Research Council Project Grant (Grant Number 453628) to R.J.N., T.E.H. and W.D.T.; National Health and Medical Research Council Peter Doherty Fellowship to T.E.H.; University of Adelaide Faculty of Health Sciences Divisional Scholarship to A.R.T.
Conflict of interest
The authors do not have any conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tuck, A.R., Mottershead, D.G., Fernandes, H.A. et al. Mouse GDF9 decreases KITL gene expression in human granulosa cells. Endocrine 48, 686–695 (2015). https://doi.org/10.1007/s12020-014-0335-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0335-6